|
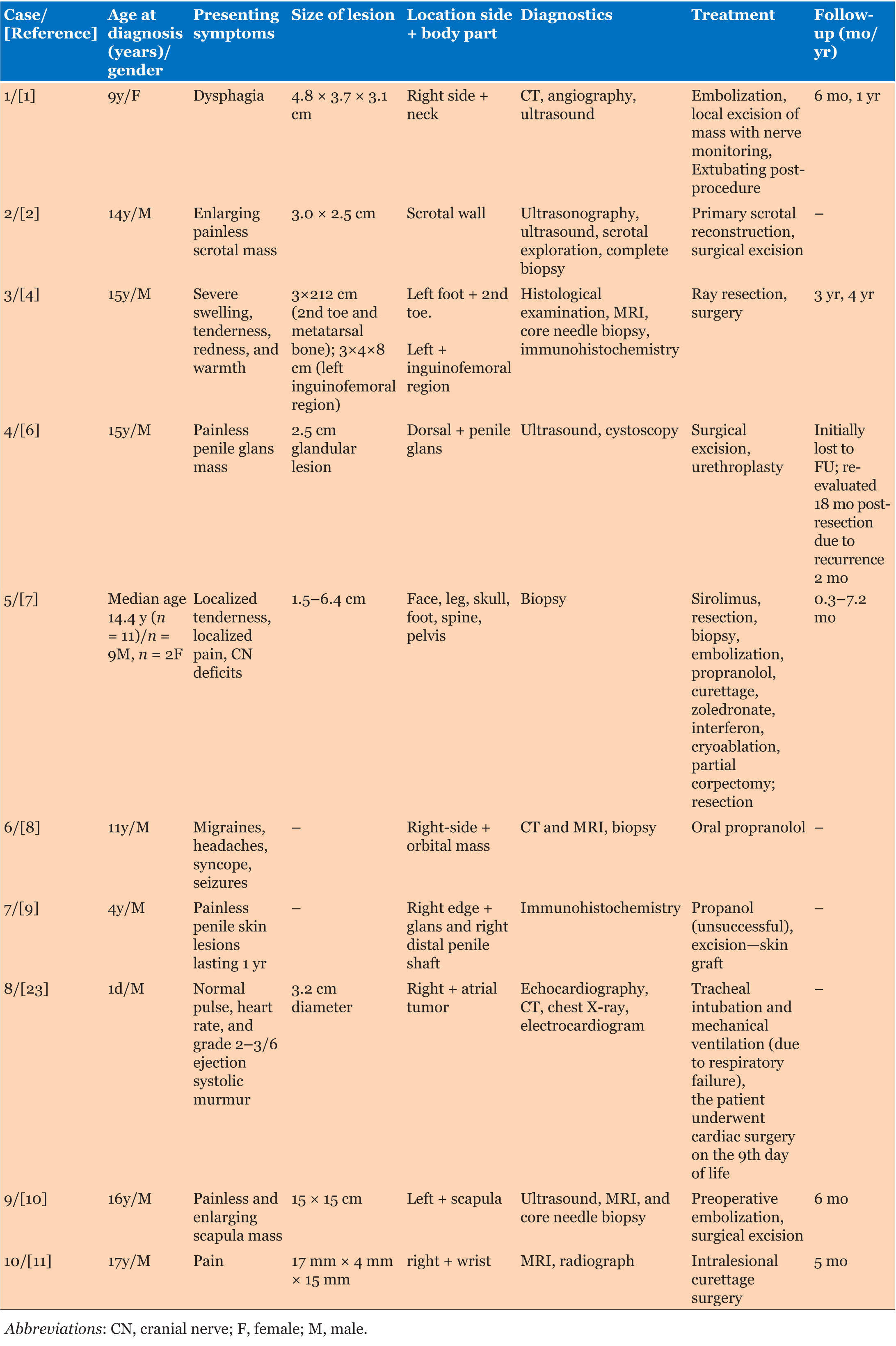
Case Report
Primary pulmonary malignant melanoma versus metastatic melanoma with indeterminate primary site
1 Resident, Department of Pathology and Molecular Medicine, McMaster University, Hamilton, Ontario, Canada
2 Associate Professor, Department of Pathology and Molecular Medicine, McMaster University, Hamilton, Ontario, Canada
3 Associate Professor, Department of Surgery, Division of Thoracic Surgery, McMaster University, Hamilton, Ontario, Canada
4 Professor, Department of Pathology and Molecular Medicine, McMaster University, Hamilton, Ontario, Canada
Address correspondence to:
Harpreet Rai
Department of Pathology and Molecular Medicine, McMaster University, 1280 Main Street West, Hamilton, Ontario L8S 4L8,
Canada
Message to Corresponding Author
Article ID: 100062Z11HR2022
Access full text article on other devices

Access PDF of article on other devices

How to cite this article
Rai H, Naqvi AH, Hanna WC, Alowami SO. Primary pulmonary malignant melanoma versus metastatic melanoma with indeterminate primary site. J Case Rep Images Pathol 2022;8:100062Z11HR2022.ABSTRACT
Introduction: Primary malignant melanoma of the lung is an extremely rare entity with a dismal prognosis.
Case Report: Herein, we present a case of primary malignant melanoma of the lung in a 67-year-old woman with a history of chest infection and a suspicious lesion on a chest X-ray. Computed tomography scan of the chest confirmed a nodule in the left lower lobe. Positron emission tomography showed a hypermetabolic tumor with no evidence of metastatic disease or mediastinal lymphadenopathy. The patient subsequently underwent surgery. Histopathological examination, supported by immunohistochemical analysis, was in accordance with malignant melanoma.
Conclusion: Since the published data focusing on cases of primary malignant melanoma of the lung are limited, the pathological features of these melanomas, their histogenesis, clinical behavior, and the possible therapeutic options are not well established.
Keywords: BRAF, Lung, Malignant melanoma
Introduction
Malignant melanoma is primarily a cutaneous malignancy. Although rare, primary extracutaneous melanomas involving the ocular region, leptomeninges, and mucosal surfaces such as the oral cavity, anorectal region, genitourinary tract, and liver have been reported [1],[2],[3],[4]. Primary malignant melanoma of lung (PMML) accounts for 0.01% of all primary lung malignancies and 0.4% of all malignant melanomas. Based on a literature search, there have been 76 cases of PMML identified [5].
Case Report
A 67-year-old woman with no smoking history presented with symptoms of a chest infection. Her past medical history was significant for a right-sided stage II invasive ductal carcinoma grade II, estrogen receptor (ER)/progesterone receptor (PR) positive, and HER2 negative. She was treated with a partial mastectomy, radiation, and ongoing antiestrogen therapy with anastrozole. She had regular follow-up with her medical team. There was no prior history of skin, ocular, or gastrointestinal (GI) lesions. Her family history was significant for breast cancer and lung cancer. There was no family history of skin cancer(s).
Her chest X-ray demonstrated a lesion in the left lower lobe (LLL) measuring 1.6 × 1.2 cm. Subsequent CT scan of the chest further confirmed a centrally located nodule in the lung. Her positron emission tomography (PET) scan showed hypermetabolic activity in the tumor. Computed tomography scan for staging showed no evidence of thoracic or intra-abdominal metastasis. Pulmonary function tests revealed an FEV1 of 102% predicted and diffusing capacity of carbon monoxide (DLCO) of 105% predicted. The patient underwent left lower lobe segmentectomy with thoracic lymphadenectomy with curative intent. A post-surgery CT scan of the chest revealed complete excision with no evidence of mediastinal or hilar lymphadenopathy.
The specimen was submitted for routine histopathological examination. Sections were stained with hematoxylin and eosin (H&E) and analyzed. Immunohistochemistry (IHC) and molecular analyses were also performed. Tumor cells were pleomorphic with high nuclear/cytoplasmic ratio, dispersed chromatin, and some with prominent nucleoli (Figure 1 and Figure 2). Tumor cells were positive for S100, HMB45, melanoma cocktail, and vimentin (Figure 3 and Figure 4), while cytokeratin, ER, PR, BRST-2, mammoglobin, GATA3, TTF-1, and Napsin-A were all negative. Histopathological analysis supported by IHC led to a diagnosis of malignant melanoma with negative resection margins. There was no lymph-vascular invasion and no nodal metastasis. Molecular analysis was negative for BRAF mutations.
After the diagnosis of malignant melanoma, the patient was examined thoroughly with no clinical evidence of cutaneous, ocular, or mucosal melanoma. She subsequently developed a solitary brain metastasis involving the frontal lobe and was transferred under palliative care. She passed away 11 months after the initial diagnosis of PMML.
Discussion
Melanoma is a malignancy of melanocytes, pigment producing cells which originate from the neural crest and are present in the basal epidermis, hair follicles, most squamous mucous membranes, and leptomeninges [6],[7],[8]. Cutaneous melanomas are unique in that they can spontaneously regress after metastasis; therefore, it is difficult to recognize the difference between a PMML and a metastatic lesion to the lung. Malignant melanomas involving the lung are nearly always metastatic until proven otherwise. Before establishing the diagnosis of PMML, other sites of origin such as skin, eyes, and mucosal surfaces must be ruled out.
Diagnostic criteria have evolved with increased understanding of PMML [9]. According to the Armed Forced Institute of Pathology, the presence of an intraepithelial component within the bronchial epithelium (which is a part of the original criteria by Allen and Drash [10]) supports a primary pulmonary origin, should not be required or considered pathognomonic for the diagnosis of PMML. This is because of the known occurrence of intraepithelial growth of metastatic malignant melanoma to the lung. The selection criteria proposed by Armed Forces Institute of Pathology involves: (1) A solitary tumor; (2) A malignant melanoma confirmed by immunohistochemistry and/or electron microscopy; (3) No past history of excision or fulguration of a cutaneous, mucous membrane, or ocular lesion; (4) A central pulmonary lesion; (5) No demonstrable tumor elsewhere at the time of diagnosis [9]. The patient in our case study fulfilled the above criteria of PMML.
The exact histogenesis of PMML is not well understood. Several theories have been proposed but challenge remains: (1) Migration of benign melanocytes during embryogenesis. This theory is supported by the presence of melanocytes and melanocytic proliferation in the esophagus and larynx, which shares a common embryonic origin with the lung [10]. (2) Melanogenic metaplasia in the submucosal bronchial glands, which is speculated to occur in response to chronic irritation and squamous metaplasia. (3) Melanoma cells may be derived from pluripotent stem cells in the lower respiratory tract. There have been previous reports of melanocytic differentiation in neuroectodermal and neuroendocrine neoplasms [11]. (4) Some have speculated that these lung lesions are metastatic malignant melanoma with spontaneous regression of previous skin lesions [12].
Non-specific clinical features make a clinical diagnosis of PMML challenging. Primary malignant melanoma of lung may present as an incidental finding or with non-specific respiratory symptoms. The most common presenting symptoms are cough, hemoptysis, chest pain, dyspnea, and weight loss. In 30% of patients, there may be an incidental finding on imaging [13]. Primary malignant melanoma of lung mainly affects middle aged individuals as compared to lung cancer which affects older individuals. Based on the literature review by Paliogiannis et al., approximately half of PMML patients in whom data regarding smoking habits was available were non-smokers, which suggest that there might be a different etiopathogenesis for PMML than lung cancer.
As PMML is considered a non-UV related melanoma, it might exhibit few somatic mutations unlike sun-exposed cutaneous melanomas. Non-cutaneous melanomas have significantly lower numbers of mutations [14]. Molecular analysis with BRAF is important in management of cutaneous melanomas. From a literature search, one case of BRAF mutation [15] and one case of NRAS mutation [16] have been reported in PMML, which is not enough data to draw substantial conclusions. We performed genetic testing on our patient and no BRAF mutations were detected.
Given the rarity of PMML, there are no clear guidelines from the National Comprehensive Cancer Network (NCCN) regarding treatment. In practice, surgical resection of the tumor with an adequate margin and lymph node dissection represent the treatment regimen of choice. In patients with locally advanced or distant metastatic disease, chemotherapy with dacarbazine and immunotherapy with interleukin-2 or interferon are considered [17].
In recent years, discovery of BRAF mutations in cutaneous malignant melanoma (MM) patients has led to treatment with BRAF inhibitors, such as vemurafenib and dabrafenib, together with an MEK inhibitor such as trametinib [18],[19]. Immunotherapy with programmed death-1 (PD-1) checkpoint inhibitors has shown promise in metastatic cutaneous MM [20]. Even though several new agents have been approved for the treatment of cutaneous MM, there is a paucity of published information regarding the efficacy and safety of these combinations in other melanoma subtypes. Further studies are required to establish a definitive role for these modalities in the treatment of PMML.
Overall, the 5-year survival rate of all patients with lung cancer, irrespective of stage, is 16%, compared to a 5-year survival rate of stage 4 melanoma metastatic to the lung of 7–9% [21]. For PMML, there is insufficient data due to the rarity of the disease to establish a 5-year survival rate. However, based on published case reports, the prognosis of PMML is poor with occasional long-term survivors [22].
Conclusion
Despite the absence of melanocytes in the lung, cases of PMML have been observed. The clinical differentiation between PMML and other lung cancers is challenging. Histopathological examination supported by IHC is the mainstay of diagnosis along with radiological and clinical criteria. Although rare, PMML should be considered in the differential diagnosis of primary tumors of the lung.
REFERENCES
1.
La Selva D, Kozarek RA, Dorer RK, Rocha FG, Gluck M. Primary and metastatic melanoma of the GI tract: Clinical presentation, endoscopic findings, and patient outcomes. Surg Endosc 2020;34(10):4456–62. [CrossRef]
[Pubmed]

2.
Barillaro F, Camilli M, Dessanti P, et al. Primary melanoma of the bladder: Case report and review of the literature. Arch Ital Urol Androl 2018;90(3):224–6. [CrossRef]
[Pubmed]

3.
Sutton B, Chan R, Sutton M, Boone T. Primary malignant melanoma of the genitourinary tract with upper and lower tracts involvement. Case Rep Urol 2013;2013:217254. [CrossRef]
[Pubmed]

4.
Du F, Yang M, Fang J, Jing C. Primary hepatic malignant melanoma: A case report. Int J Clin Exp Pathol 2015;8(2):2199–201.
[Pubmed]

5.
Paliogiannis P, Fara AM, Pintus G, et al. Primary melanoma of the lung: A systematic review. Medicina (Kaunas) 2020;56(11):576. [CrossRef]
[Pubmed]

6.
Fitzpatrick TB. The biology of pigmentation. Birth Defects Orig Artic Ser 1971;7(8):5–12.
[Pubmed]

7.
Schaffer JV, Bolognia JL. The biology of the melanocyte. In: Cancer of the Skin. Elsevier; 2011. p. 23–39. [CrossRef]

8.
Pomeranz AA, Garlock JH. Primary melanocarcinoma of the esophagus. Ann Surg 1955;142(2):296–301. [CrossRef]
[Pubmed]

9.
Wilson RW, Moran CA. Primary melanoma of the lung: A clinicopathologic and immunohistochemical study of eight cases. Am J Surg Pathol 1997;21(10):1196–202. [CrossRef]
[Pubmed]

10.
Allen MS Jr, Drash EC. Primary melanoma of the lung. Cancer 1968;21(1):154–9. [CrossRef]
[Pubmed]

11.
Jennings TA, Axiotis CA, Kress Y, Carter D. Primary malignant melanoma of the lower respiratory tract. Report of a case and literature review. Am J Clin Pathol 1990;94(5):649–55. [CrossRef]
[Pubmed]

12.
Smith JL Jr, Stehlin JS Jr. Spontaneous regression of primary malignant melanomas with regional metastases. Cancer 1965;18(11):1399–415. [CrossRef]
[Pubmed]

13.
Ost D, Joseph C, Sogoloff H, Menezes G. Primary pulmonary melanoma: Case report and literature review. Mayo Clin Proc 1999;74(1):62–6. [CrossRef]
[Pubmed]

14.
Davis EJ, Johnson DB, Sosman JA, Chandra S. Melanoma: What do all the mutations mean? Cancer 2018;124(17):3490–9. [CrossRef]
[Pubmed]

15.
Landgraf LG, Malias MA, Patterson SJ. Primary melanoma of the lung treated with surgery, dabrafenib and trametinib. Case Rep Oncol 2020;13(2):789–92. [CrossRef]
[Pubmed]

16.
Hibiya T, Tanaka M, Matsumura M, et al. An NRAS mutation in primary malignant melanoma of the lung: A case report. Diagn Pathol 2020;15(1):11. [CrossRef]
[Pubmed]

17.
Bajetta E, Del Vecchio M, Bernard-Marty C, et al. Metastatic melanoma: Chemotherapy. Semin Oncol 2002;29(5):427–45. [CrossRef]
[Pubmed]

18.
Ascierto PA, Minor D, Ribas A, et al. Phase II trial (BREAK-2) of the BRAF inhibitor dabrafenib (GSK2118436) in patients with metastatic melanoma. J Clin Oncol 2013;31(26):3205–11. [CrossRef]
[Pubmed]

19.
Flaherty KT, Infante JR, Daud A, et al. Combined BRAF and MEK inhibition in melanoma with BRAF V600 mutations. N Engl J Med 2012;367(18):1694–703. [CrossRef]
[Pubmed]

20.
Yun S, Vincelette ND, Green MR, Wahner Hendrickson AE, Abraham I. Targeting immune checkpoints in unresectable metastatic cutaneous melanoma: A systematic review and meta-analysis of anti-CTLA-4 and anti-PD-1 agents trials. Cancer Med 2016;5(7):1481–91. [CrossRef]
[Pubmed]

21.
Balch CM, Gershenwald JE, Soong SJ, et al. Final version of 2009 AJCC melanoma staging and classification. J Clin Oncol 2009;27(36):6199–206. [CrossRef]
[Pubmed]

22.
Mahowald MK, Aswad BI, Okereke IC, Ng T. Long-term survival after pneumonectomy for primary pulmonary malignant melanoma. Ann Thorac Surg 2015;99(4):1428–30. [CrossRef]
[Pubmed]

SUPPORTING INFORMATION
Author Contributions
Harpreet Rai - Conception of the work, Design of the work, Acquisition of data, Analysis of data, Drafting the work, Revising the work critically for important intellectual content, Final approval of the version to be published, Agree to be accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved.
Asghar H Naqvi - Analysis of data, Revising the work critically for important intellectual content, Final approval of the version to be published, Agree to be accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved.
Waël C Hanna - Analysis of data, Drafting the work, Final approval of the version to be published, Agree to be accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved.
Salem O Alowami - Conception of the work, Design of the work, Acquisition of data, Analysis of data, Drafting the work, Revising the work critically for important intellectual content, Final approval of the version to be published, Agree to be accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved.
Guarantor of SubmissionThe corresponding author is the guarantor of submission.
Source of SupportNone
Consent StatementWritten informed consent was obtained from the patient for publication of this article.
Data AvailabilityAll relevant data are within the paper and its Supporting Information files.
Conflict of InterestAuthors declare no conflict of interest.
Copyright© 2022 Harpreet Rai et al. This article is distributed under the terms of Creative Commons Attribution License which permits unrestricted use, distribution and reproduction in any medium provided the original author(s) and original publisher are properly credited. Please see the copyright policy on the journal website for more information.