|
Case Report
Next-generation sequencing proves clonal relationship between two distinguished lung and liver carcinomas by standard histopathology approach
1 Department of Medicine, State University of New York Downstate Health Science University, Brooklyn, NY 11203, USA
2 Department of Pathology, State University of New York Downstate Health Science University, Brooklyn, NY 11203, USA
3 Division of Gastroenterology and Hepatology, State University of New York Downstate Health Science University, Brooklyn, NY 11203, USA
4 Banner MD Anderson Cancer Center, Banner University Medical Center, Phoenix, AZ 85006, USA
Address correspondence to:
Shivakumar Vignesh
Banner MD Anderson Cancer Center, Banner University Medical Center, Phoenix, AZ 85006,
USA
Message to Corresponding Author
Article ID: 100064Z11TL2022
Access full text article on other devices

Access PDF of article on other devices

How to cite this article
Li T, Diks J, Nguyen ST, Zeng J, Chen N, Vignesh S. Next-generation sequencing proves clonal relationship between two distinguished lung and liver carcinomas by standard histopathology approach. J Case Rep Images Pathol 2022;8(2):6–11.ABSTRACT
Introduction: Two tumors having different histopathologies at anatomically distinct sites giving the picture of dual primary malignancies. Here we presented a case of two possible primary tumors and one secondary mass.
Case Report: A 74-year-old female, active smoker, without personal or family cancer history presented with early satiety and weakness for two months. Systems review was positive for a “raw” feeling in stomach, alleviated with antacids. Vital signs were stable with a negative abdominal exam. Lab showed leukocytosis 24.8 K/uL (3.5–10.8 K/uL) with left shift, microcytic anemia with hemoglobin 6.1 g/dL (12.0–16.0 g/dL), and reactive thrombocytosis 477 K/uL (130–400 K/uL). Contrast-enhanced computed tomography (CT) showed right upper lobe necrotizing cavitating lesion with reactive mediastinal and right hilar lymphadenopathy, two irregular hypodense lesions in pancreatic head and tail without ductal dilation with two irregular hypodense liver lesions. Immunohistochemistry of lung and pancreatic lesions were biopsied through endoscopic ultrasound (EUS), consistent with poorly differentiated squamous cell carcinoma (SCC) with extensive necrosis, which indicates pancreatic masses are likely metastases from the lung. Liver lesion biopsy exhibited high-grade neuroendocrine tumor (NET) with focal necrosis. Next gene sequencing was pursued. Given poor functional status, palliative immunotherapy was offered; however, the patient succumbed to respiratory failure.
Conclusion: Given the morphology and immunoprofile, differential diagnosis includes dual primary cancers with one metastasis, or primary SCC with metastasis with neuroendocrine differentiation. Despite having different histopathology and immunophenotype, both lung and liver tumors harbor the same molecular profile even at the variants of unknown significance that show identical mutations. As a result, they are directly related. TP53, RB1, MYCL1, and MEK1 mutations are more prevalent in SCC than NET. Tumor mutation burden values may vary as the tumor clonal structure varies between primary and metastatic sites, with higher rates of monoclonal structure recorded in metastases due to clonal selection, leading to a reduction in overall genetic diversity (“bottlenecking”). This raises the suspicion that the liver tumor is a SCC with neuroendocrine differentiation. The paucity of the specimen and rapid clinical course limited further investigation. Germline testing would have been useful to determine whether these findings are somatic or germline.
Keywords: Genetic predisposition, Liver neuroendocrine tumor, Lung non-small cell carcinoma, Multiple primaries, Neoplasms, Pancreatic carcinoma
Introduction
The presence of multiple primary cancers in a single patient was first reported more than 100 years back. Since then, this phenomenon has been identified with increasing frequency, in part due to the increased life expectancy of cancer survivors—a boon of advancements in cancer therapies, long-term side effects of chemotherapy, and/or radiation therapy, increased screening sensitivity, and persisting effects of genetic and behavioral risk factors.
The two cancers are either detected at the same time (synchronous) or one may follow the other after a period of time (metachronous). A synchronously existing primary involving both organs in a single patient is a rarity in the medical literature.
Case Report
A 74-year-old female presented to the hospital due to generalized weakness for two weeks, poor appetite, and early satiety for two months. Review of system was positive for a “raw” feeling in the stomach, which was mildly alleviated with antacids. The patient denied any abdominal pain, vomiting, change of bowel habits, or weight loss. The patient was not able to identify the color of stool due to bilateral blindness.
The patient’s past medical history included hypertension, type 2 diabetes mellitus, coronary artery disease status post 5 stents 12 years ago, bilateral blindness, chronic obstructive pulmonary disease, and morbid obesity. Past surgical history was cholecystectomy. Social history was significant for life-long active smoking. No family history of cancer was reported. Vital signs were unremarkable with blood pressure 96/62 mmHg, heart rate 67 bpm, respiratory rate 19 breaths per minute, and afebrile. The abdomen was non-distended and non-tender with no mass appreciated. Rectal exam showed green mucoid unformed stool.
Complete blood count showed leukocytosis white blood cells (WBC) 24.75 K/uL (normal range: 3.5–10.8 K/uL) with left shift, severe microcytic anemia with hemoglobin 6.1 g/dL (normal range: 12.0–16.0 g/dL), and reactive thrombocytosis 477 K/uL (normal range: 130–400 K/uL). Comprehensive metabolic panels were unremarkable with normal liver function test results. Iron studies revealed a picture of anemia due to chronic disease (Table 1).
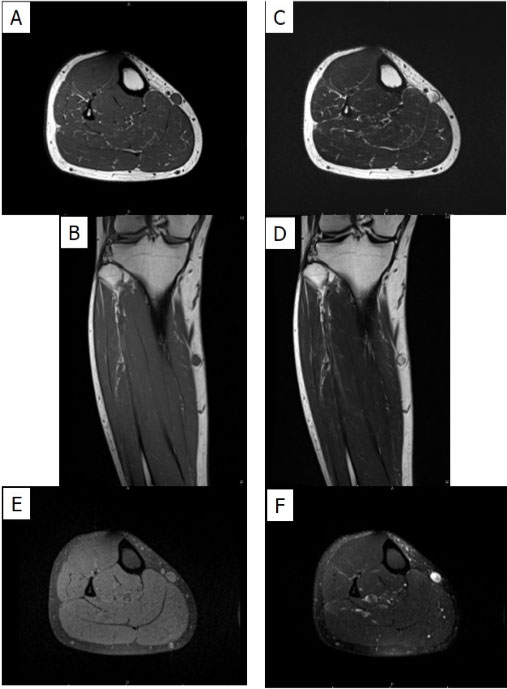
Computed tomography (CT) of the chest with intravenous (IV) contrast showed a right upper lobe necrotizing cavitating lesion measuring 7.2 × 7.2 6.5 cm in three dimensions associated with reactive mediastinal and right hilar lymphadenopathy (LAD) (Figure 1A). Computed tomography of the abdomen and pelvis with IV contrast revealed two irregular hypodense lesions in the pancreas, one appears to be originating from the pancreatic head measuring 3.3 × 4.8 3.7 cm, and another 8.9 × 6.2 × 4.6 cm necrotic mass in the tail, without pancreatic ductal dilation (Figure 1B). Meanwhile, two irregular hypodense lesions were incidentally spotted in Couinaud segment 7⁄8 and 5 of the right hepatic lobe, measuring 3.3 × 2.6 cm and 1.4 ×1.5 cm respectively (Figure 1C).
Later on, endoscopic ultrasound illustrated complex solid-cystic, hypoechoic, heterogenous with central anechoic areas in pancreas uncinate process measuring 29 × 31 mm without evidence of communication with or obstruction of the main pancreatic duct (Figure 1D). However, left lobe of the liver had multiple hypoechoic lesions with hyperechoic rim concerning metastatic lesions (Figure 1E).
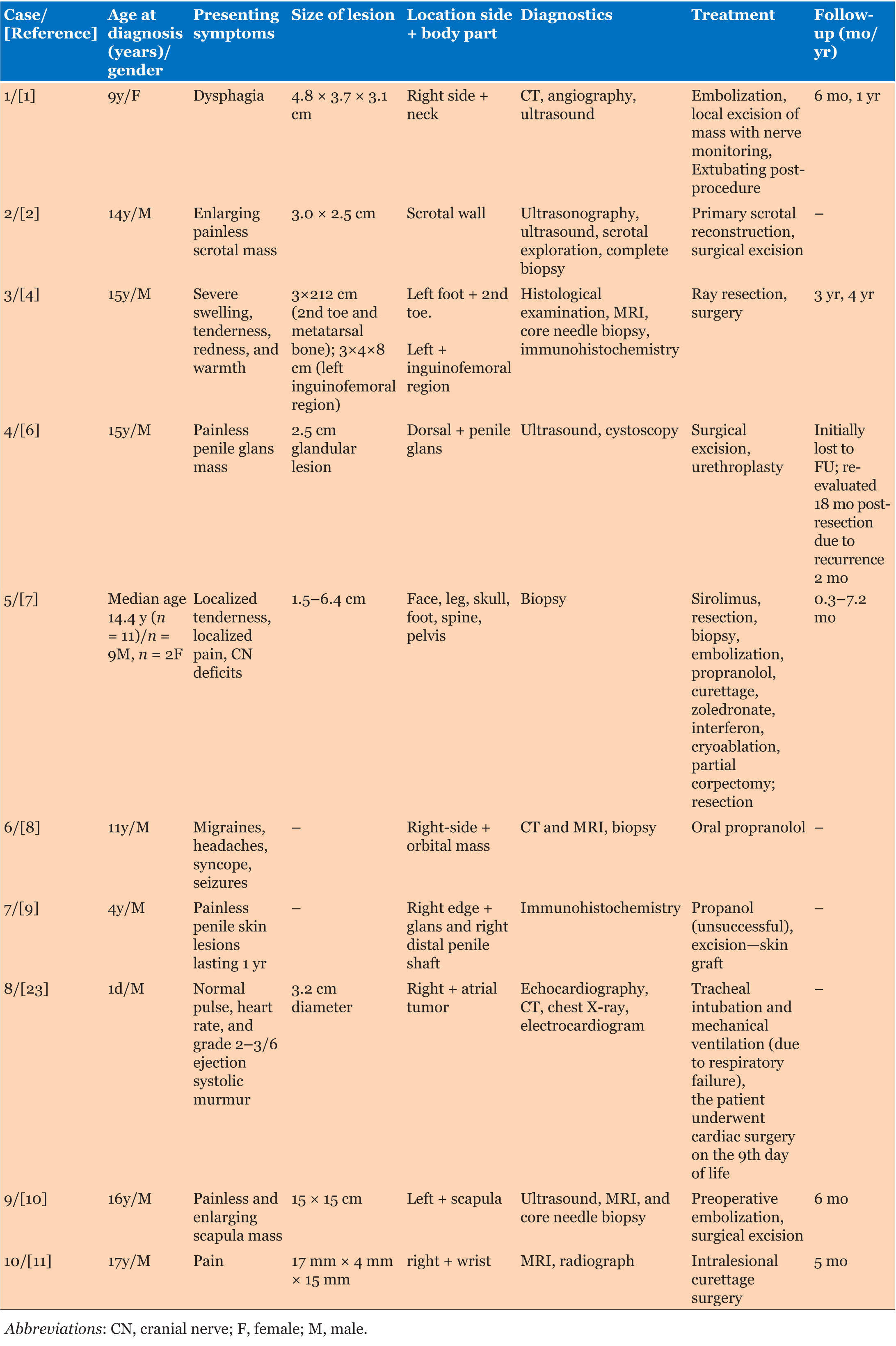
Fine needle biopsy (FNA) and immunohistochemical studies of the lung via endobronchial ultrasound and pancreatic head lesion were consistent with similar morphology of poorly differentiated squamous cell carcinoma with extensive necrosis (Figure 1F and Figure 1G). Whereas, left liver lobe FNA demonstrated high-grade neuroendocrine tumor with focal necrosis, intermediate mitoses (focally 10 mitotic counts per 10 HPF) and Ki-67 rate 60–70% (Figure 1H) with immunohistochemical studies showing caudal-type homeobox transcription factor 2 (CDX2) positivity and negative thyroid transcription factor 1 (TTF-1) (Table 2). In order to investigate the genetics underlying the three different histological tumors including a large 7 cm cavitated lung mass with hilar LAD, a 4 cm pancreatic uncinate process mass and multiple liver lesions, next gene sequencing of lung, and liver samples were pursued with result showing mitogen-activated protein kinases (the MAPK/ERK kinase) MAP2K1 (MEK1) C121S subclonal with Rictor and Mycl1 amplification, RB1 and TP53 alteration (Table 3). Tumor mutational burden in lung and liver were 10/Mb and 6/Mb, respectively, indicating liver was second primary malignancies instead of metastases from lung or pancreas.
Given poor functional status and multiple comorbidities, chemotherapy was deferred. Immunotherapy was compassionately offered after functional status was improved. Unfortunately, the patient subsequently developed large pleural effusion and melena within two months of disease onset. In order to honor the patient and her family’s wishes, further interventions including endoscopy and resuscitation were deferred. Given poor functional status, palliative immunotherapy was offered; however, the patient succumbed to respiratory failure.
Discussion
The occurrence of multiple primary cancers in particular individuals has intrigued clinicians and scientists for more than a century and was first described in 1879 by Billroth [1],[2],[3]. Most multiple primary cancers are double primary cancers. The neoplasms may be limited to a single organ or involve multiple anatomically distinct organs.
Warren and Gates criteria (1932) are commonly used to propose a diagnosis of multiple primary malignancies with requirement of the following: (a) each tumor should present a definite picture of malignancy; (b) each tumor should be histologically distinct; (c) the possibility that one is a metastasis of the other must be excluded [3].
The two most common used definitions for multiple malignancies are the Surveillance Epidemiology and End Results (SEER) Program used by the North American Association of Central Cancer Registries (NAACCR) and the International Association of Cancer Registries and International Agency for Research on Cancer (IACR/IARC) used internationally. The SEER Program classifies multiple primary tumors into two categories based on a two-month period to distinguish synchronous and metachronous depending on the timeline of two cancer occurrence [4], whereas IARC/IACR suggests a 6-month period [5][6].
Depending on definition, overall reported frequency of multiple primary cancers varies between 2.4% and 17% [7]. Metachronous primary malignancies are becoming increasingly common because of an increase life expectancy of cancer survivors, greater awareness with improved diagnostic and treatment modalities. In comparison, synchronous tumors occur uncommonly, with the most common site for synchronously existing multiple tumors being the breast. An autopsy series has reported prostate cancer as one of the most common malignancies in patients with multiple primary cancers and also as a frequent incidental autopsy finding in elderly men [8]. First primary malignant tumors (PMTs) were most common in the hematopoietic system and the cervix; second PMTs were most common in lungs and the hematopoietic systems. Second PMTs were less well differentiated than index PMTs [8].
The pathophysiology behind the occurrence of multiple primary malignancies has been theorized to be common carcinogen induced multiple cancers in an exposed epithelial surface, called “field cancerization,” genetic and environmental predisposition to neoplasia [9]. Significant temporal changes have been found in the prevalence of cancer, risk factors such as smoking and obesity in our patient as well as advances in diagnostic sensitivity and improved screening program that lead to more discovery of second or more cancers [10]. The epidemiological factors of multiple primary malignancy includes genetic susceptibility, environmental and lifestyle influences, hormonal factors, immune deficiency and infection, carcinogenic effects resulted from previous cancer treatment, and finally, interaction among all of above factors, leading to cancer development [11],[12],[13].
In patients with lung cancer, the incidence of multiple primaries ranges from 13.4% to 22% [14]. Many second malignancies are related to smoking. Among lung cancer, small cell lung cancer (SCLC) has the highest rate of secondary primaries with 4.46 cases per 100 person-years followed by squamous cell carcinoma and adenocarcinoma with a rate of 3.77 per 100 person-years versus 3.36, respectively [7]. There is no significant association with radiotherapy [15]. The most common second cancer with index lung cancer is within the lung, especially if the first one was a SCLC. Colorectal and bladder cancers are commonly seen as extra-thoracic second malignancies, possibly due to common vulnerability to smoking [7].
Back to our patient, the differential diagnosis would be three primary malignancies or three tumors originating from stem mutation or epigenetic difference caused by genetic aberrations. Pancreatic masses are likely metastases from the lung primary or vice versa, thus could not constitute two individual primary tumors based on the definition, meanwhile a multiple primary cancer history was reported to be a common condition among pancreatic cancer [16]. Blood gene sequence needs to be done to prove the stem mutation proposal. Last but not least, different accumulated mutational burdens in two highly differentiated tumors—lung squamous carcinoma and liver neuroendocrine tumor excluded the hypothesis of molecular aberrations causing different morphologies in two tumors. Double primary cancer with one metastasis is a more reasonable diagnosis in this case with three tumors. This assumption also aligned with the NAACCR definition and Warren and Gates criteria that multiple lesions of different histologic types occurring in different sites are considered as separate primaries whether occurring simultaneously or at different time [3],[4].
In a nutshell, multiple primary malignancies are rare entities and this case enforces the possible diagnosis of multiple primary malignancies in patients with high tumor burden. Management of those patients should be individualized based on multidisciplinary collaboration. Further research efforts are warranted to better understand, recognize thus prevent the development of multiple primary malignancies.
Conclusion
In a nutshell, the coexistence of squamous cell carcinoma (SCC) and NET in the one patient is exceedingly rare and might indicate NET may arise from SCC via neuroendocrine differentiation. The paucity of the specimen and rapid course of events limit the ability for further investigation. Germline testing would have been useful to distinguish whether the findings in this patient (TP53 and RB1) are somatic or germline.
REFERENCES
1.
Stenhouse G, Fyfe N, King G, Chapman A, Kerr KM. Thyroid transcription factor 1 in pulmonary adenocarcinoma. J Clin Pathol 2004;57(4):383–7. [CrossRef]
[Pubmed]

2.
Werling RW, Yaziji H, Bacchi CE, Gown AM. CDX2, a highly sensitive and specific marker of adenocarcinomas of intestinal origin: An immunohistochemical survey of 476 primary and metastatic carcinomas. Am J Surg Pathol 2003;27(3):303–10. [CrossRef]
[Pubmed]

3.
4.
5.
International Association of Cancer Registries. International rules for multiple primary cancers. Asian Pac J Cancer Prev 2005;6(1):104–6.
[Pubmed]

6.
Working Group Report. International rules for multiple primary cancers (ICD-0 Third edition). Eur J Cancer Prev 2005;14(4):307–8. [CrossRef]
[Pubmed]

7.
Vogt A, Schmid S, Heinimann K, Frick H, Herrmann C, Cerny T, Omlin A. Multiple primary tumours: Challenges and approaches, a review. ESMO Open 2017;2(2):e000172. [CrossRef]
[Pubmed]

8.
Lee TK, Myers RT, Scharyj M, Marshall RB. Multiple primary malignant tumors (MPMT): Study of 68 autopsy cases (1963–1980). J Am Geriatr Soc 1982;30(12):744–53. [CrossRef]
[Pubmed]

9.
Slaughter DP, Southwick HW, Smejkal W. Field cancerization in oral stratified squamous epithelium; clinical implications of multicentric origin. Cancer 1953;6(5):963–8.
[Pubmed]

10.
Copur MS, Manapuram S. Multiple primary tumors over a lifetime. Oncology (Williston Park) 2019;33(7): 629384.
[Pubmed]

11.
Wood ME, Vogel V, Ng A, Foxhall L, Goodwin P, Travis LB: Second malignant neoplasms: Assessment and strategies for risk reduction. J Clin Oncol 2012;30(30):3734–45. [CrossRef]
[Pubmed]

12.
13.
Supramaniam R. New malignancies among cancer survivors: SEER cancer registries, 1973–2000. Journal of Epidemiology & Community Health 2008;62(4):375–6. [CrossRef]

14.
Rosso S, De Angelis R, Ciccolallo L, et al. Multiple tumours in survival estimates. Eur J Cancer 2009;45(6):1080–94. [CrossRef]
[Pubmed]

15.
Sánchez de Cos Escuín J, Rodríguez López DP, Utrabo Delgado I, Gallego Domínguez R, Sojo González MA, Hernández Valle M. Disease recurrence and second tumors in long-term survivors of lung cancer. Arch Bronconeumol 2016;52(4):183–8. [CrossRef]
[Pubmed]

16.
Gerdes B, Ziegler A, Ramaswamy A, Wild A, Langer P, Bartsch DK. Multiple primaries in pancreatic cancer patients: Indicator of a genetic predisposition? Int J Epidemiol 2000;29(6):999–1003. [CrossRef]
[Pubmed]

SUPPORTING INFORMATION
Author Contributions
Tian Li - Acquisition of data, Analysis of data, Drafting the work, Revising the work critically for important intellectual content, Final approval of the version to be published, Agree to be accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved.
John Diks - Analysis of data, Drafting the work, Revising the work critically for important intellectual content, Final approval of the version to be published, Agree to be accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved.
Snow Trinh Nguyen - Analysis of data, Revising the work critically for important intellectual content, Final approval of the version to be published, Agree to be accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved.
Jianying Zeng - Analysis of data, Revising the work critically for important intellectual content, Final approval of the version to be published, Agree to be accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved.
Neil Chen - Analysis of data, Revising the work critically for important intellectual content, Final approval of the version to be published, Agree to be accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved.
Shivakumar Vignesh - Conception of the work, Design of the work, Revising the work critically for important intellectual content, Final approval of the version to be published, Agree to be accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved.
Guarantor of SubmissionThe corresponding author is the guarantor of submission.
Source of SupportNone
Consent StatementWritten informed consent was obtained from the patient for publication of this article.
Data AvailabilityAll relevant data are within the paper and its Supporting Information files.
Conflict of InterestAuthors declare no conflict of interest.
Copyright© 2022 Tian Li et al. This article is distributed under the terms of Creative Commons Attribution License which permits unrestricted use, distribution and reproduction in any medium provided the original author(s) and original publisher are properly credited. Please see the copyright policy on the journal website for more information.