|
Case Series
Rare secondary lymphomas involving the brain
1 Department of Pathology and Laboratory Medicine, Donald and Barbara Zucker School of Medicine at Hofstra/Northwell, 2200 Northern Blvd, Suite 104, Greenvale, NY, USA
Address correspondence to:
Pallavi Khattar
MD, Department of Pathology and Laboratory Medicine, Donald and Barbara Zucker School of Medicine at Hofstra/Northwell, 2200 Northern Blvd, Suite 104, Greenvale, NY 11548,
USA
Message to Corresponding Author
Article ID: 100082Z11ZZ2024
Access full text article on other devices

Access PDF of article on other devices

How to cite this article
Hossein-Zadeh Z, Geetha S, Seth N, Edappallath S, Bedi P, Zhang X, Li JY, Khattar P. Rare secondary lymphomas involving the brain. J Case Rep Images Pathol 2024;10(2):27–31.ABSTRACT
Central nervous system (CNS) involvement is rare in patients with Hodgkin lymphoma (HL). Accordingly, the clinical features and outcomes are not well described. Anaplastic large cell lymphoma (ALCL) is a rare and aggressive type of non-Hodgkin lymphoma that can arise in various parts of the body, including the central nervous system (CNS). Secondary ALCL involving the CNS is more common than primary disease. In general, these rare lymphomas in the CNS present with a predilection for parenchymal lesions with dural extension. Here we report two cases with metastatic/secondary lymphomas involving the CNS, with review of the literature. We believe awareness of these rare entities involving the CNS is beneficial for pathologists and neuropathologists.
Keywords: Anaplastic large cell lymphoma, CNS lymphoma, Hodgkin lymphoma, Metastatic brain lymphoma, Primary versus metastatic CNS lymphomas
Introduction
Most systemic lymphomas can cause concomitant central nervous system (CNS) involvement; however, CNS involvement is more frequent at the time of relapse. The median time from systemic diagnosis to CNS disease is approximately one year, although many cases can present within six months or less from the time of diagnosis [1]. Although any CNS compartment can be involved, leptomeningeal spread is the most common presentation, seen in as many as 6–8% of patients with non-Hodgkin lymphoma (NHL) [2]. Some common risk factors for CNS involvement in systemic lymphomas include aggressive subtypes of NHL, older age at diagnosis, advanced stage, and high International Prognostic Index, as well as testicular, orbital or paranasal sinus involvement [3]. Burkitt, lymphoblastic, and intravascular lymphomas are associated with CNS involvement in up to 50% cases and require CNS prophylaxis [4],[5]. Additionally, double-hit lymphomas characterized by co-expression of MYC and B-cell lymphoma 2 protein (BCL2) and/or BLC6 rearrangements have a risk of 4–7% of CNS involvement at diagnosis, and a 3-year cumulative risk of 13% [6].
Central nervous system involvement by Hodgkin lymphoma (HL) is rare and often has a dismal prognosis. Unlike NHL, HL rarely involves the CNS. The incidence has been reported as few as 0.2–0.5% of all HL cases. In a comprehensive review by Re et al. of 14,868 patients, only 2 patients were identified to have CNS HL [7]. Most reports consist of a single case or small case series highlighting the limited knowledge about the clinical characteristics, prognosis, and management of this rare CNS complication [8]. Furthermore, there is no consensus to guide therapeutic decision-making. Although the overall 5-year survival rate for patients with CNS involvement by HL is quite low, a better prognosis is possible in patients who achieved a complete response to treatment, particularly those who present with CNS involvement or involvement of the CNS is the sole site of disease relapse [8],[9].
Anaplastic large cell lymphoma (ALCL) is an uncommon T-cell lymphoma comprised of large, pleomorphic, CD30-positive cells [10]. Most cells express T-cell antigens, including CD2, CD3, CD4, and other T-cell-defining antigens. Central nervous system involvement of ALCL is an extremely rare finding, either as secondary spread of primary-nodal/systemic ALCL or as primary CNS ALCL [11]. The majority of ALCL harbor the anaplastic lymphoma kinase-1 (ALK-1) t(2;5) translocation and only a minority of ALCL are ALK-1 negative, and in both instances CNS involvement is exceedingly rare [12]. In this case series, we present 2 cases of rare secondary lymphomas with metastasis to the CNS along with the review of the literature. The first is a case of metastatic HL, and the second is a case of a metastatic ALK-negative, ALCL. Based on the review of the literature we present the first case series of CNS involvement by these mentioned diagnoses in elderly adult patients.
Case Report
Case 1
A 75-year-old female presented with intermittent headaches over a one-week period, accompanied by vertigo and diplopia. She did not have any history of fevers, chills, numbness, paresthesia, or weakness. Additionally, there were no recent incidents of falls, trauma, or changes in medications. Upon clinical examination, she displayed a faint, fatigable horizontal nystagmus in both eyes. Examination of other systems yielded normal results.
Her history is significant for well-controlled hypertension (HTN), gastro-esophageal reflux disease, unspecified arrhythmia status post ablation, and nodular sclerosis-type HL which was diagnosed four years earlier and successfully treated with the AAVD regimen. A repeat positron emission tomography-computed tomography (PET-CT) performed last year revealed fludeoxyglucose-18 (FDG) avid lesions in the right and left acetabulum, L5 vertebral body, and left sacral wing. A biopsy of the left sacral wing lesion was negative for lymphoma.
Her current condition was worked up with imaging studies. An initial CT scan of the brain revealed a right cerebellar hematoma with surrounding edema, mass effect on the fourth ventricle, and secondary obstructive hydrocephalus. However, a CT angiogram did not reveal any vascular malformations. Subsequently, a magnetic resonance imaging (MRI) was performed, confirming the presence of a subacute hemorrhage in the right cerebellar region. Considering the patient’s age, hypertension history, and lesion location, a vascular bleed was considered the primary differential diagnosis. However, MR spectroscopy results revealed high levels of lipids, suggestive of a neoplastic process, most likely a lymphoma. Given the patient’s prior diagnosis of HL, an extremely rare but possible occurrence of HL in the brain was also considered. Computed tomography scans of the abdomen, pelvis, and chest were obtained to look for any evidence of metastatic disease, but it did not reveal any lesions. Therefore, a plan was made to perform a stereotactic biopsy of the cerebellar lesion, with a preliminary frozen section diagnosis. Initially, two biopsy samples were taken, but they did not reveal any neoplastic cells. However, on the third pass of the cerebellar lesion, a few atypical large cells were identified on the frozen section. Subsequently, the remainder of the biopsy was collected from this site as well as frontal lobe and sent for a permanent examination.
Pathologic findings
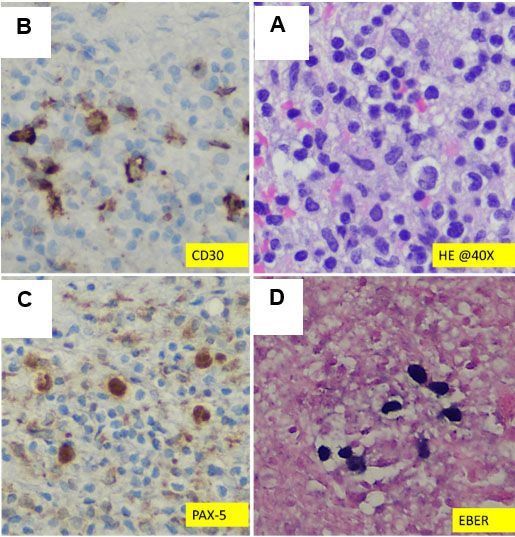
Microscopic examination revealed large atypical mononuclear lymphoid cells consistent with Hodgkin cells, including mummified cells and lacunar cells, as shown in Figure 1 and Figure 2. The background consisted of small lymphocytes, plasma cells, histiocytes, and eosinophils, with no evidence of necrosis. Immunohistochemical stains were performed, demonstrating strong positivity for CD30, MUM1, and p53 in the large, atypical HL cells. These cells exhibited weak PAX5 positivity (Figure 2) and were negative for CD3, CD20, BOB1, EMA, SOX10, CAM5.2, CD5, BCL2, BCL6, CyclinD1, CD21, and CD23. Additionally, in situ Epstein–Barr virus (EBV) hybridization testing showed strong positivity in the Hodgkin cells (Figure 1). The proliferation index (Ki-67) was moderate in the neoplastic cells and low in the microenvironment (Figure 2). Based on these findings, a diagnosis of classic HL involving cerebellar tissue was established.
Case 2
The chart is limited regarding this patient; however, in summary, the patient was a deceased 85-year-old female with a past medical history of HTN, hypothyroidism, arthritis, and basal cell carcinoma of the lip, with a recent diagnosis of ALCL involving the ear. She initially presented with altered mental status, syncopal events, and an abnormal gait. Imaging showed multiple foci of hypoechoic lesions in the frontal mass, for which the patient underwent stereotactic biopsies, and the results were consistent with T-cell anaplastic large cell lymphoma (ALK-negative). Given the patient’s recent diagnoses of anaplastic large cell lymphoma of the ear, the brain findings are most likely a metastatic process rather than a primary disease.
Discussion
Leptomeningeal metastases (LM) from solid tumors, lymphoma, and leukemia are characterized by multifocal neurological deficits with a high mortality rate. However, a definitive diagnosis is not always possible as 25% of cerebrospinal fluid samples result in false-negative reports at first cytological examination [13]. Detecting CNS metastasis in patients with malignancies is critical to monitoring disease status, electing appropriate therapy, and providing prognosis, but can be challenging [14]. In general, primary CNS lymphomas are uncommon, making up only 2% of primary brain tumors. Furthermore, the vast majority of primary CNS lymphomas are of B-cell lineage, mainly diffuse large B cell lymphoma [15].
In comparison to non-Hodgkin lymphomas, CNS involvement is exceedingly rare in patients with Hodgkin lymphoma, nonetheless, the clinical features and outcomes are not well described. Our current knowledge and understanding of CNS-HL is limited and derived from single reports or small case series, and the optimal therapeutic approach and course of disease after CNS involvement is unknown [2]. Intracranial Hodgkin’s disease at presentation is even more uncommon with only 8 reported cases. Most cases of intracranial involvement by Hodgkin’s disease occur at the time of relapse. The most common presenting feature of intracranial Hodgkin’s disease is a cranial nerve palsy with brain parenchyma being the most common intracranial site of involvement. Mixed cellularity histology is the most frequent subtype of Hodgkin’s disease among these patients [3].
In a multicenter international retrospective analysis, by Gerstner and colleagues, the authors aimed to describe the incidence, clinical characteristics, pathologic features, responses to therapy, and outcomes of patients diagnosed with CNS-HL in a large multicenter retrospective study of 16 patients diagnosed with CNS-HL. The median overall survival for all 16 patients was 60.9 months from first diagnosis of HL (systemic or CNS) and 43.8 months from diagnosis of CNS-HL. Central nervous system-Hodgkin lymphoma was discovered simultaneously with or prior to systemic HL in 6 patients. There are no known risk factors for the development of CNS spread in patients with HL. A role for immunosuppression and EBV infection in systemic HL as well as in primary CNS-HL has been suggested; however, due to limited number of CNS-HL cases in addition to limited EBV testing of the sample tissue it is difficult to interpret a specific role of EBV in CNS-HL. Although a majority of patients have died, long-term survival is possible in patients who achieve a complete response to treatment, particularly those who present with CNS involvement or involvement of the CNS is the sole site of relapse [8].
Anaplastic large cell lymphoma is a rare and aggressive type of non-Hodgkin lymphoma that can arise in various parts of the body, including the central nervous system (CNS). Anaplastic large cell lymphomas typically harbor demonstrable anaplastic lymphoma kinase (ALK) gene rearrangement with resultant ALK protein expression; ALK-negative ALCL is much less common [16]. Secondary CNS T-cell lymphoma generally affects about 5% of patients with T- or NK-cell lymphoma, with some exceptions. Anaplastic lymphoma kinase-positive ALCL with extranodal involvement >1 also has high risk of CNS progression 12. It is observed that, ALK expression in ALCL patients is generally associated with younger age and a favorable outcome with overall survival of 71–83% [17].
Conclusion
Involvement of the CNS by lymphomas is most commonly seen in diffuse large B-cells lymphomas as well as acute B or T-cell leukemias/lymphomas. Here we report two cases of rare secondary CNS lymphomas in a patient with a prior known history of classic Hodgkin lymphoma and another patient with a history of anaplastic large cell lymphoma. Central nervous system (CNS) involvement is rare in patients with Hodgkin lymphoma (HL). In general, these rare lymphomas in the CNS present with a predilection for parenchymal lesions with dural extension. We believe awareness of these secondary CNS involvement by these rare types of lymphomas is beneficial for pathologists and neuropathologists.
REFERENCES
1.
Bernstein HM, English C, Young RB, Venugopal S. A 70-year-old man with relapsed CNS lymphoma has incidental finding of right atrial mass. Chest 2022;162(1):e43–8. [CrossRef]
[Pubmed]

2.
Taylor JW, Flanagan EP, O’Neill BP, et al. Primary leptomeningeal lymphoma: International Primary CNS Lymphoma Collaborative Group report. Neurology 2013;81(19):1690–6. [CrossRef]
[Pubmed]

3.
Boehme V, Zeynalova S, Kloess M, et al. Incidence and risk factors of central nervous system recurrence in aggressive lymphoma—A survey of 1693 patients treated in protocols of the German High-Grade Non- Hodgkin’s Lymphoma Study Group (DSHNHL). Ann Oncol 2007;18(1):149–57. [CrossRef]
[Pubmed]

4.
Cheah CY, Bröckelmann PJ, Chihara D, et al. Clinical characteristics and outcomes of patients with Hodgkin lymphoma with central nervous system involvement: An international multicenter collaboration. Am J Hematol 2016;91(9):894–9. [CrossRef]
[Pubmed]

5.
Shimada K. Treatment strategy for central nervous system involvement in intravascular large B-cell lymphoma. [Article in Japanese]. Brain Nerve 2011;63(5):467–72.
[Pubmed]

6.
Rosenthal A, Younes A. High grade B-cell lymphoma with rearrangements of MYC and BCL2 and/or BCL6: Double hit and triple hit lymphomas and double expressing lymphoma. Blood Rev 2017;31(2):37–42. [CrossRef]
[Pubmed]

7.
Weihrauch MR, Re D, Scheidhauer K, et al. Thoracic positron emission tomography using 18F-fluorodeoxyglucose for the evaluation of residual mediastinal Hodgkin disease. Blood 2001;98(10):2930–4. [CrossRef]
[Pubmed]

8.
Gerstner ER, Abrey LE, Schiff D, et al. CNS Hodgkin lymphoma. Blood 2008;112(5):1658–61. [CrossRef]
[Pubmed]

9.
Hirmiz K, Foyle A, Wilke D, et al. Intracranial presentation of systemic Hodgkin’s disease. Leuk Lymphoma 2004;45(8):1667–71. [CrossRef]
[Pubmed]

10.
Yang T, Belverud S, Yeh AY, et al. Primary CNS anaplastic diffuse large B-cell lymphoma mimicking undifferentiated metastatic tumors: A case report. J Neurooncol 2010;96(3):433–6. [CrossRef]
[Pubmed]

11.
Wang CX, Wang H, Li J, et al. Brain metastasis of ALK positive anaplastic large cell lymphoma after a longterm disease free survival in an old adult. Int J Clin Exp Pathol 2014;7(3):1182–7.
[Pubmed]

12.
Pang Y, Chihara D. Primary and secondary central nervous system mature T- and NK-cell lymphomas. Semin Hematol 2021;58(2):123–9. [CrossRef]
[Pubmed]

13.
Galicia N, Díez P, Dégano RM, Guest PC, Ibarrola N, Fuentes M. Proteomic biomarker identification in cerebrospinal fluid for leptomeningeal metastases with neurological complications. Adv Exp Med Biol 2017;974:85–96. [CrossRef]
[Pubmed]

14.
Kimura A, Takemura M, Serrero G, et al. Higher levels of progranulin in cerebrospinal fluid of patients with lymphoma and carcinoma with CNS metastasis. J Neurooncol 2018;137(3):455–62. [CrossRef]
[Pubmed]

15.
Yuan C, Duan H, Wang Y, Zhang J, Ou J, Wang W, Zhang M. Primary central nervous system ALKnegative anaplastic large cell lymphoma: A case report and literature review. Ann Palliat Med 2022;11(4):1554–60. [CrossRef]
[Pubmed]

16.
Brady AL, Fuller CE, Patel S, Hall W, Banki K, Ghimire KB. Primary CNS ALK-negative anaplastic large cell lymphoma: A case report and review of the literature. Radiol Case Rep 2023;19(1):393–9. [CrossRef]
[Pubmed]

17.
Rannan-Eliya YF, Pulford K, Johnson R, et al. Isolated cutaneous anaplastic large cell lymphoma progressing to severe systemic disease with myocardial involvement and central nervous system infiltration. Pediatr Blood Cancer 2008;50(4):879–81. [CrossRef]
[Pubmed]

SUPPORTING INFORMATION
Author Contributions
Zarrin Hossein-Zadeh - Acquisition of data, Drafting the work, Final approval of the version to be published, Agree to be accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved.
Saroja Geetha - Acquisition of data, Drafting the work, Final approval of the version to be published, Agree to be accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved.
Neha Seth - Acquisition of data, Drafting the work, Final approval of the version to be published, Agree to be accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved.
Sushmitha Edappallath - Acquisition of data, Drafting the work, Final approval of the version to be published, Agree to be accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved.
Puneet Bedi - Acquisition of data, Revising the work critically for important intellectual content, Final approval of the version to be published, Agree to be accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved.
Xinmin Zhang - Analysis of data, Revising the work critically for important intellectual content, Final approval of the version to be published, Agree to be accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved.
Jian Yi Li - Acquisition of data, Analysis of data, Revising the work critically for important intellectual content, Final approval of the version to be published, Agree to be accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved.
Pallavi Khattar - Conception of the work, Design of the work, Acquisition of data, Analysis of data, Drafting the work, Revising the work critically for important intellectual content, Final approval of the version to be published, Agree to be accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved.
Guarantor of SubmissionThe corresponding author is the guarantor of submission.
Source of SupportNone
Consent StatementWritten informed consent was obtained from the patient for publication of this article.
Data AvailabilityAll relevant data are within the paper and its Supporting Information files.
Conflict of InterestAuthors declare no conflict of interest.
Copyright© 2024 Zarrin Hossein-Zadeh et al. This article is distributed under the terms of Creative Commons Attribution License which permits unrestricted use, distribution and reproduction in any medium provided the original author(s) and original publisher are properly credited. Please see the copyright policy on the journal website for more information.