|
Case Report
Primary retroperitoneal mucinous cystic tumor with borderline malignancy, microinvasion, and KRAS mutation: A case report
1 Resident, Service of Anatomic Pathology and Cytopathology, University Hospital “Dr. José Eleuterio González”, Monterrey, Nuevo León, Mexico
2 Head of Service and Pathology Professor, Service of Anatomic Pathology and Cytopathology, University Hospital “Dr. José Eleuterio González”, Monterrey, Nuevo León, Mexico
3 Pathology Professor, Service of Anatomic Pathology and Cytopathology, University Hospital “Dr. José Eleuterio González”, Monterrey, Nuevo León, Mexico
Address correspondence to:
Mauricio Gonzalo Alanis Arredondo
Decápolis #805, Col. Jardines de San Agustín, San Pedro Garza García, Nuevo León,
Mexico
Message to Corresponding Author
Article ID: 100083Z11MA2024
Access full text article on other devices

Access PDF of article on other devices

How to cite this article
Arredondo MGA, Quintana OB, Meza IAO. Primary retroperitoneal mucinous cystic tumor with borderline malignancy, microinvasion, and KRAS mutation: A case report. J Case Rep Images Pathol 2024;10(2):32–37.ABSTRACT
Introduction: Primary retroperitoneal mucinous cystic tumors with borderline malignancy are rare neoplasms of uncertain histopathogenesis with few cases reported in the literature. Histologically, these tumors are an analogous counterpart to the more common ovarian mucinous borderline tumor. A microinvasive component of these neoplasms may contribute to higher probabilities of recurrence and poor prognosis, while the presence of various mutations, particularly in KRAS and GNAS exons, could have therapeutic relevance in the future.
Case Report: Imaging studies of a 42-year-old female with abdominal pain and distension revealed a large abdominopelvic cystic tumor. This tumor was initially misdiagnosed as adnexal. Gynecologic surgery showed the tumor to depend on the retroperitoneum. The patient had intact gynecologic organs. Further, a macroscopic study revealed a cystic cavity filled with serous liquid, with dominant papillary excrescence, and multiple microscopic papillae distributed along the inner lining. Moreover, a histologic analysis showed a proliferation of more than 10% of cylindrical mucosecretory epithelium, with dysplasia and focal infiltration to the stroma. Additionally, the immunohistochemistry revealed diffuse epithelial positivity for CK7 and focal CK20 positivity. Superior and inferior gastrointestinal endoscopies were done, where the possibility of a neoplastic origin in here was ruled out. Next-generation sequencing showed a missense mutation in exon 2 of KRAS.
Conclusion: While the histopathogenesis of primary retroperitoneal mucinous tumors remains uncertain, the overall behavior and prognosis is remarkably similar to their better-known mucinous counterparts in the ovary. The possibility of targeted treatments in cases with malignancy, recurrence, and metastases also remains an important topic in molecular oncology, highlighting the need for further molecular profiling.
Keywords: Borderline, Mucinous, Retroperitoneal, Tumor
Introduction
Primary retroperitoneal mucinous cystic tumors with borderline malignancy (PRMT-BM) are rare neoplasms of uncertain histopathogenesis, with few cases reported in the literature. Histologically, PRMT-BM is an analogous counterpart to the more common ovarian mucinous borderline tumor. The presence of a microinvasive component in these neoplasms may contribute to higher probabilities of recurrence and a poor prognosis, while the presence of various mutations, particularly in KRAS and GNAS exons, could become of therapeutic relevance in the future; thus, further research is necessary to understand the histopathogenesis, prognosis, and molecular profiles of these rare neoplasms.
Case Report
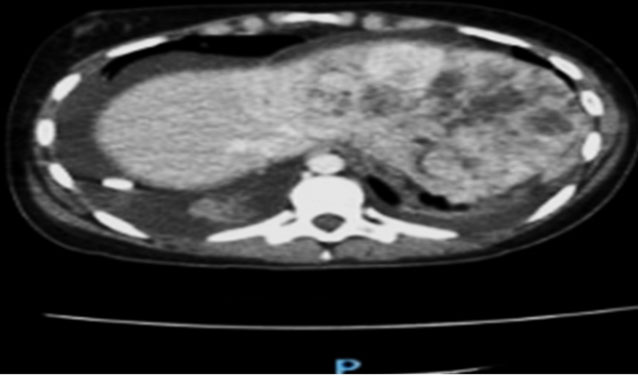
A 42-year-old female presented with a progressive increasement of volume on the right flank. She eventually noticed a painless mobile mass, which was accompanied by changes in the voiding pattern, including polyuria. She initially consulted a physician, who ordered an abdominal ultrasound and tomography (CT) scan. The ultrasound revealed a simple cyst of unknown origin, measuring of 16 cm in diameter, while the computed tomography (CT) scan identified the same lesion as a peritoneal inclusion cyst, measuring 19 cm in diameter. Consequently, she was referred to a specialized hospital.
She was initially assessed by gynecologists, who decided to verify the diagnosis by conducting serum tumor marker tests, which returned within normal parameters. Additionally, a transvaginal ultrasound was performed, which indicated a probable left anexial cystadenoma. Consequently, they decided to do an exploratory laparotomy and found that the mass originated from the retroperitoneum, while the reproductive organs were found to be intact. Therefore, the gynecologists decided to end the surgery and refer her to the general surgery department.
General surgeons decided to do an enhanced abdominopelvic CT scan, which revealed a thin-walled ovoid lesion with well-defined borders and content with a liquid density of 5–10 HU and some calcifications. This lesion was described as being retroperitoneal in origin, with a diameter of 18.7 cm and an approximate volume of 2000 cc, which displaced adjacent structures. Additionally, they identify a delay in the nephrogram of the right kidney and hydronephrosis. Notably, the reproductive organs and digestive system did not have alterations. Based on these findings, the general surgeons proceeded with surgery to remove the entirety of the tumor. No additional eventualities were reported. The specimen was then sent for a histopathologic evaluation.
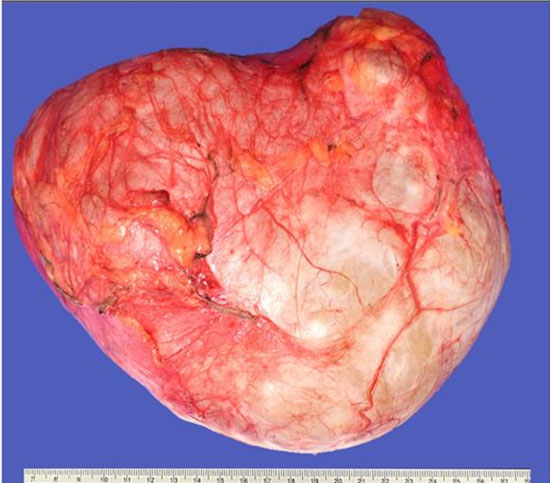
Gross examination revealed a cystic tumor weighing 2 kg and measuring 20 cm in diameter, with a lobulated morphology, smooth shiny surface partially covered by fibroadipose tissue. The tumor was soft to the touch (Figure 1). The inner lining was mostly smooth, except for a dominant excrescence of 2.2 cm in diameter and multiple punctiform papillae distributed throughout the inner surface (Figure 2). The entirety of the dominant excrescence and several of the smaller papillae were submitted for further analysis.
Histopathological examination revealed a cystic neoplasm composed of a fibrous capsule lined on the inside by a cuboidal simple epithelium, alternating with multiple areas of a proliferated cylindrical mucosecretory epithelium, characterized by pseudostratification of the nuclei, fold formations, focal pseudoinvasion, and focal true infiltrative microinvasion. The microinvasive component measured 2 mm in total, arising from a few foci of severely dysplastic epithelium, where small clusters of cells could be seen invading the fibrous stroma beneath it (Figure 6). The rest of the fibrous stroma was hypocellular, with few inflammatory cells and was otherwise unremarkable (Figure 3, Figure 4, Figure 5, Figure 6).
Immunohistochemistry staining was performed for CK7 and CK20. The former revealed diffuse positivity in the epithelium while the latter only showed focal positivity. Furthermore, CA-125 and CDX2 were done, with the former exhibiting diffuse positivity in the invasive component and the latter exhibiting negativity, with only focal positivity in the non-invasive epithelium (Figure 7, Figure 8, Figure 9, Figure 10).
After the histopathological diagnosis, the patient was referred to gastroenterology to assess the possibility of a primary origin in the gastrointestinal tract. Superior endoscopy and colonoscopy were performed, revealing only atrophic gastritis and external hemorrhoids, with no evidence of a neoplasm.
Next-generation sequencing (Thermo Fisher Ion Torrent Genexus System), a technique used for DNA and RNA sequencing and mutation detection, was performed on the tumor, which detected one missense mutation involving p.G12D (c.35G>A) in exon 2 of the KRAS gene.
The patient had no formal follow-up with her oncologist, but she is still alive and reported to be asymptomatic ever since she recovered from the surgery.
Discussion
Primary retroperitoneal mucinous tumors (PRMTs) were initially described in 1924 by Handfield-Jones in his study on retroperitoneal cysts [1]. They can be defined as mucosecretory epithelial neoplasms arising in the adipose tissue of the retroperitoneum with no association to organs. The cystic variations of these neoplasms are classified as cystadenomas (benign), borderline malignancies (intermediate), and cystadenocarcinomas (malignant). According to Wolf et al.’s case series, the most common of these PRMTs are mucinous cystadenocarcinomas, while the least common are the borderline malignancies [2].
Perhaps the most interesting aspect of these neoplasms is their uncertain histopathogenesis. While PRMTs present more frequently in female patients, their occurrence in males widens the etiological possibilities. Therefore, several theories of origin have been proposed, mainly: metaplasia of celomic tissue, implantation of ectopic ovarian tissue, enterogenic duplication cysts, and monodermal teratomas [3],[4].
The diagnostic approach of PRMTs can be difficult. Although imaging techniques are sensitive in the detection of various cystic lesions with low, intermediate, and high malignancy potential, specificity in these techniques is lacking. Primary retroperitoneal mucinous tumors can mimic a variety of different diagnoses, making histopathological classification an imperative to reach a proper diagnosis [5]. Even though aspiration is an effective technique for identifying the characteristics of a cyst, the cytological examination of the aspirated fluid often falls short in determining the kind of epithelial cells that line the cyst [6].
Most patients present either asymptomatic or with vague symptoms, possibly with a palpable mass, distention, or pain [3].
Serum tumor markers, such as carcinoembryonic antigen; carbohydrate antigen CA19.9, CA15.3, CA125; and alpha fetoprotein have proven neither specific nor sensitive in the diagnosis or follow-up of these neoplasms [7].
The primary treatment of PRMT-BM is surgical excision, either laparoscopic or open, generally with similar survival rates as mucinous cystadenomas even though PRMTs with borderline malignancy show higher rates of recurrence [2]. While the laparoscopic approach offers a relatively less invasive procedure and faster recovery time, it remains controversial whether it increases the risk of tumor implantation and recurrence [8]. Conversely, the open approach offers the surgeon more maneuverability and better tumor handling, theoretically reducing the possibility of spillage and implantations.
Generally, there is no need for adjuvant therapy after successful removal of a PRMT-BM, with close follow-ups regarded as adequate for post-surgical management. However, the use of chemotherapy is controversial, with some authors recommending it be reserved to cases with malignancy, tumor rupture, recurrence, or metastases [9].
Histologically, PRMTs consist of one or more cystic spaces lined with mucosecretory epithelium that can range in feature from benign, reactive, and borderline to malignant. Additionally, a non-secretory cuboidal or flat epithelium resembling mesothelium may be present. The stroma is fibrous and can vary from hypocellular to hypercellular, with or without an ovary-like stroma, usually with some degree of inflammatory cell activity.
The borderline form of PRMTs is characterized by a proliferation of the epithelium, causing pseudostratification and tufting, with mild to moderate dysplasia. Moreover, the borderline form can progress to carcinoma in situ, characterized by a non-invasive epithelium with severe dysplasia, overt nuclear atypia, increased nuclear-cytoplasmic ratio, loss of polarity, and mitoses. The significance of microinvasion compared to overt invasion is not well understood. Nevertheless, true invasion must be distinguished from pseudoinvasion.
The immunohistochemical profile of PRMTs is mostly non-specific and inconsistent, as seen in multiple case reports. The epithelium is mostly CK7 positive, with variable positivity for CK20, CEA, CA125, CA19-9, CDX2, MUC2, calretinin, and WT-1. Similarly, both epithelium and stroma can show variable positivity for estrogen and progesterone receptors.
According to The Cancer Genome Atlas database, KRAS-G12D, G12V, G12C, and G13D are the most common KRAS mutations in carcinomas. Further, G12D is found in 18 types of cancer, including lung, colon, and pancreatic cancers.
While the molecular findings in our case report caused no immediate change in the oncologic treatment of the patient, they contributed to the identification of the oncogenic drivers of these rare neoplasms, which could become relevant in the future as potentially viable KRAS-targeted drugs are developed. One such drug is MRTX1133, a small molecule inhibitor that targets the KRAS G12D mutation, which has shown promising results in preclinical trials in mouse models of pancreatic cancer [10],[11].
Regarding prognosis, KRAS mutations, including the variant found in this case, are known to be associated with a more aggressive clinical course in various neoplasms, including ovarian borderline tumors, where these mutations are particularly associated with invasive implants, higher stages of the disease, increased recurrence rates, and worse adverse disease-free survival compared to outcomes from wild-type KRAS borderline tumors [12],[13]. Specifically regarding the limited PRMTBM molecular landscape, a previous case report of a PRMT-BM identified a KRAS mutation in exon 3 and a GNAS mutation in exon 8 [14].
Conclusion
Unfortunately, the clinical and radiologic characteristics of PRMTs are non-specific, requiring resection for a proper diagnosis. Although their histopathogenesis is uncertain, the overall behavior and prognosis of PRMTs are remarkably similar to those of their better-known mucinous counterparts in the ovary. Exploiting the potential of targeted treatments for malignant, recurrent, and metastases cases remains an important topic in molecular oncology, highlighting the need for further molecular profiling.
REFERENCES
1.
Handfield-Jones RM. Retroperitoneal cysts: Their pathology, diagnosis, and treatment. British Journal of Surgery 1924;12(45):119–34. [CrossRef]

2.
Wolf B, Kunert C, Horn LC, Einenkel J. Management of primary retroperitoneal mucinous tumors: A retrospective meta-analysis. Int J Gynecol Cancer 2017;27(6):1064–71. [CrossRef]
[Pubmed]

3.
Roma AA, Malpica A. Primary retroperitoneal mucinous tumors: A clinicopathologic study of 18 cases. Am J Surg Pathol 2009;33(4):526–33. [CrossRef]
[Pubmed]

4.
Tomisaki I, Matsuyama A, Jotatsu M, Yamamura S, Onishi R, Fujimoto N. Primary retroperitoneal mucinous cystadenocarcinoma with transition from the mesothelium. IJU Case Rep 2020;3(4):137–40. [CrossRef]
[Pubmed]

5.
Zhang Y, Yang J, Chen Z, Sun J, Wang P. Laparoscopic resection and pre-operative imaging of primary retroperitoneal mucinous neoplasms: A retrospective case series. Cancer Manag Res 2020;12:5451–60. [CrossRef]
[Pubmed]

6.
Yang DM, Jung DH, Kim H, et al. Retroperitoneal cystic masses: CT, clinical, and pathologic findings and literature review. Radiographics 2004;24(5):1353–65. [CrossRef]
[Pubmed]

7.
Myriokefalitaki E, Luqman I, Potdar N, Brown L, Steward W, Moss EL. Primary retroperitoneal mucinous cystadenocarcinoma (PRMCa): A systematic review of the literature and meta-analysis. Arch Gynecol Obstet 2016;293(4):709–20. [CrossRef]
[Pubmed]

8.
Zhang J, Zeng Q, Kang J, Chen J, Luo G, Liang Y. Primary retroperitoneal mucinous cystic tumour of borderline malignancy mimicking kidney duplicate: Cases report and literature review. BMC Urol 2023;23(1):32. [CrossRef]
[Pubmed]

9.
Dayan D, Abu-Abeid S, Klausner JM, Sagie B. Primary retroperitoneal mucinous cystic neoplasm: Authors’ experience and review of the literature. Am J Clin Oncol 2016;39(5):433–40. [CrossRef]
[Pubmed]

10.
Kemp SB, Cheng N, Markosyan N, et al. Efficacy of a small-molecule inhibitor of KrasG12D in immunocompetent models of pancreatic cancer. Cancer Discov 2023;13(2):298–11. [CrossRef]
[Pubmed]

11.
Tang D, Kang R. Glimmers of hope for targeting oncogenic KRAS-G12D. Cancer Gene Ther 2023;30(3):391–3. [CrossRef]
[Pubmed]

12.
Zuo T, Wong S, Buza N, Hui P. KRAS mutation of extraovarian implants of serous borderline tumor: Prognostic indicator for adverse clinical outcome. Mod Pathol 2018;31(2):350–7. [CrossRef]
[Pubmed]

13.
McHenry A, Rottmann DA, Buza N, Hui P. KRAS mutation in primary ovarian serous borderline tumors correlates with tumor recurrence. Virchows Arch 2023;483(1):71–9. [CrossRef]
[Pubmed]

14.
Son SM, Woo CG, Yun SJ, Lee OJ. Primary retroperitoneal mucinous cystic neoplasm of borderline malignancy with KRAS and GNAS co-mutation: A case report. J Int Med Res 2023;51(5):3000605231172469. [CrossRef]
[Pubmed]

SUPPORTING INFORMATION
Author Contributions
Mauricio Gonzalo Alanis Arredondo - Conception of the work, Design of the work, Acquisition of data, Analysis of data, Drafting the work, Revising the work critically for important intellectual content, Final approval of the version to be published, Agree to be accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved.
Oralia Barboza Quintana - Conception of the work, Design of the work, Acquisition of data, Analysis of data, Revising the work critically for important intellectual content, Final approval of the version to be published, Agree to be accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved.
Itzel Aracely Ortiz Meza - Acquisition of data, Analysis of data, Revising the work critically for important intellectual content, Final approval of the version to be published, Agree to be accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved.
Guarantor of SubmissionThe corresponding author is the guarantor of submission.
Source of SupportNone
Consent StatementWritten informed consent was obtained from the patient for publication of this article.
Data AvailabilityAll relevant data are within the paper and its Supporting Information files.
Conflict of InterestAuthors declare no conflict of interest.
Copyright© 2024 Mauricio Gonzalo Alanis Arredondo et al. This article is distributed under the terms of Creative Commons Attribution License which permits unrestricted use, distribution and reproduction in any medium provided the original author(s) and original publisher are properly credited. Please see the copyright policy on the journal website for more information.