|
Case Report
Epithelioid hemangioma of the skull: Rare case report
1 Student, Interdisciplinary Faculty of Health Sciences, University of Ottawa, Ottawa, Ontario, Canada
2 Student, Medical Science, Western University, London, Ontario, Canada
3 Clinical Researcher, Department of Pathology, Children’s Hospital of Eastern Ontario, Canada
4 Pathologist, Department of Pathology, Children’s Hospital of Eastern Ontario, Ottawa, Ontario, Canada
5 Oncologist, Division of Hematology/Oncology, Children’s Hospital of Eastern Ontario, University of Ottawa, Ottawa, Ontario, Canada
6 Radiologist, Department of Diagnostic Imaging, Children’s Hospital of Eastern Ontario Research Institute, Ottawa, Ontario, Canada
Address correspondence to:
Nagwa Wilson
401 Smyth Rd, Ottawa, Ontario K1H 8L1,
Canada
Message to Corresponding Author
Article ID: 100084Z11AR2025
Access full text article on other devices

Access PDF of article on other devices

How to cite this article
Rashwan A, Riarh C, Tristani L, de Nanassy J, Ramphal R, Wilson N. Epithelioid hemangioma of the skull: Rare case report. J Case Rep Images Pathol 2025;11(1):1–7.ABSTRACT
Introduction: Epithelioid hemangiomas (EH) are benign vascular tumors that commonly occur in the skin, subcutis, and bone—most typically arising as nodules in the neck and head region. Pediatric cases with atypical presentations remain rare and challenging.
Case Report: The following outlines the case of a 2-year-old male who presented with a bump and swelling on the left side of his head. Imaging tests identified multiple, hyperdense, and focally ring-enhancing intra-axial lesions located mainly at the gray-white matter junction of bilateral frontal left-sided parietal, left-sided occipital, and left-sided temporal lobes. In addition, multiple lytic lesions were seen in the calvarium. Pathology confirmed EH. While one lesion required surgical resection, the remaining lesions showed spontaneous regression during follow-up.
Conclusion: This case highlights the diagnostic and management challenges associated with pediatric EH involving the head, including intracranial involvement. The patient’s unique presentation, age, and lesion involution expand the understanding of EH beyond its typical manifestation.
Keywords: Epithelioid hemangioma, Pediatric, Skull
Introduction
Epithelioid hemangioma (EH), also known as histiocytoid hemangioma, and angiolymphoid hyperplasia with eosinophilia, is a rare and benign vascular lesion [1],[2],[3],[4],[5],[6],[7],[8],[9],[10]. It most often occurs in the head and neck region, within the bone or soft tissue [1],[2],[5],[6],[7],[8],[9],[10]. These lesions tend to be small, usually not exceeding 10 cm [10]. Epithelioid hemangioma in children lacks comprehensive knowledge regarding clinical characteristics, outcomes, and consensus on treatment [7]. Complete removal of the lesion, via surgical excision, is the commonly reported treatment, making local recurrence rare [10]. However, treatment involving incomplete resection has a reported recurrent rate of approximately 33% [10].
We present a case of EH in a 2-year-old Asian male in the left frontal bone of the skull. Diagnostic and management challenges are discussed.
Case Report
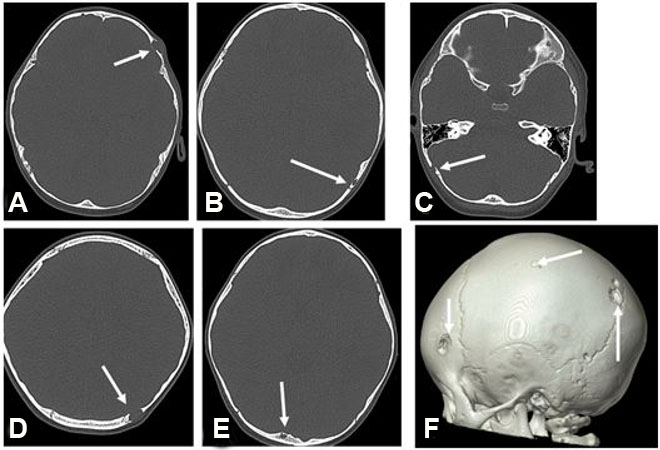
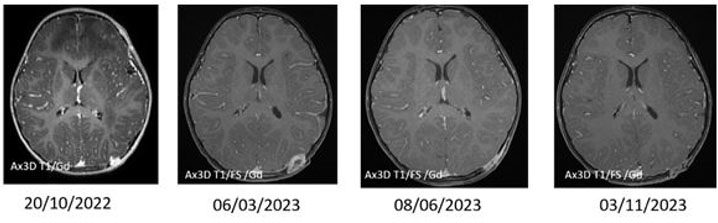
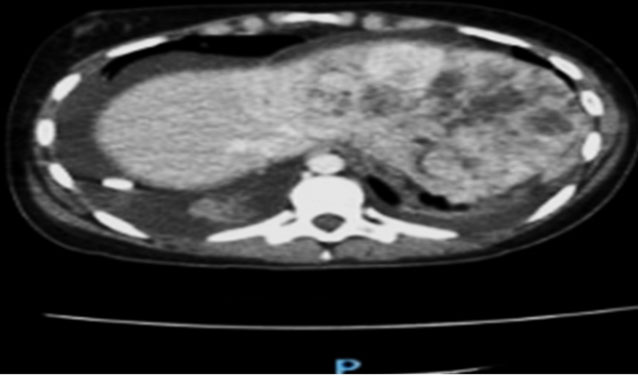
A previously healthy 2-year-old male presented with pneumonia. His workup revealed a bump on the left side of his head. He had no associated constitutional symptoms such as weight loss, fever, headaches, or seizures. He underwent imaging, including head and chest computed tomography (CT) and head magnetic resonance imaging (MRI). The latter revealed multiple ring-enhancing intra-axial lesions involving the gray-white matter junction of the bilateral frontal, left-sided parietal, left-sided occipital, and left-sided temporal lobes surrounded by significant vasogenic edema. The foci showed blooming artifact in the susceptibility weighted sequence (SWI) (Figure 1). The calvarium parietal bone showed the presence of multiple lytic intradiploic lesions associated with a soft tissue component (Figure 2). The skeletal survey did not reveal any lytic lesions. Abdominal ultrasound displayed an enlarged liver without focal lesions and no lymphadenopathy. The clinical considerations included inflammatory lesions, such as tuberculosis and fungal infection, and less likely multiple metastases. The patient is doing clinically well during follow-ups, and the MRI demonstrated improvements in intracranial lesions with no new parenchymal findings (Figure 1).
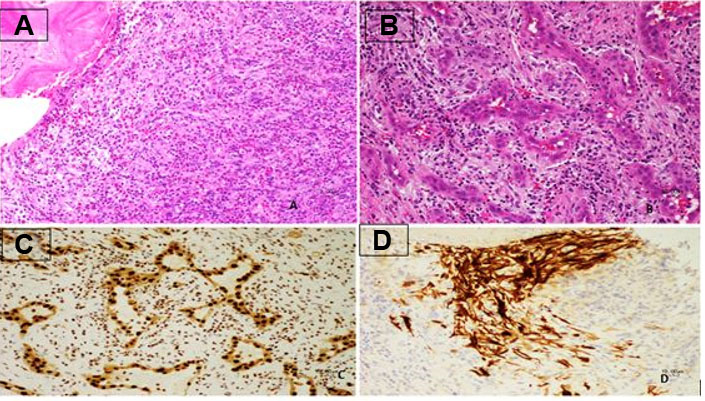
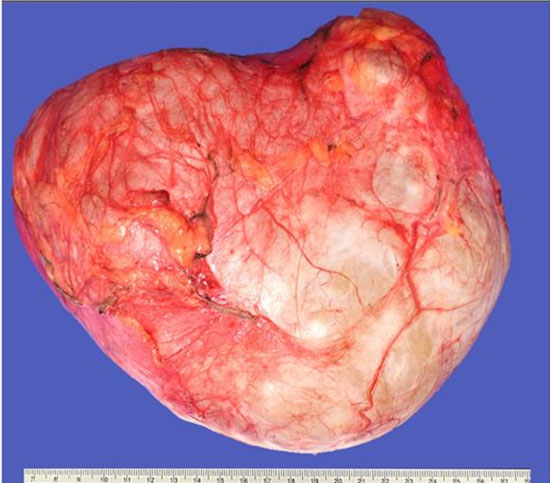
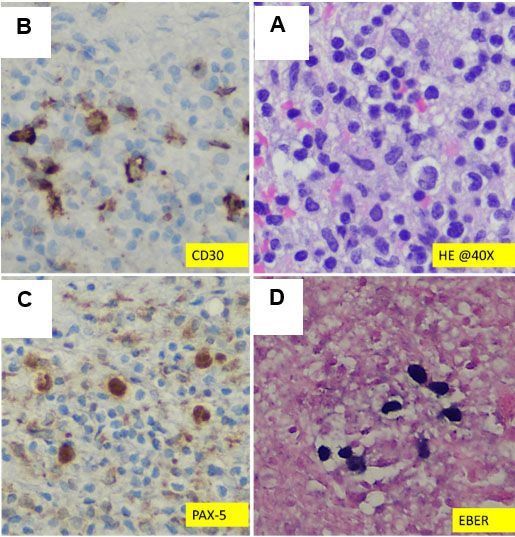
The lesion showed progressive growth during four months of follow-up; mandating a surgical resection of the lytic bony lesion with scalp soft component (Figure 3). Pathology revealed soft tissue and bone involvement with a vascular small- to medium-sized proliferation lined by endothelial cells with occasional cytoplasmic vacuolization (Figure 4). The intervening stroma showed marked reactive inflammatory cells infiltrating the background, with a predominance of eosinophils (Figure 4). Brisk mitotic activity and necrosis were not identified. Immunohistochemistry showed negative CD1a. The sarcoma panel molecular testing was negative for single-nucleotide variants (SNVs) and translocations. These findings are not commonly associated with malignant lesions, thus ruling out epithelioid hemangioendothelioma and angiosarcoma. After extensive investigation and discussion, the consensus opinion is that the morphological and immunohistochemical features seen in this skull bone lesions specimen are those of an EH. The patient underwent a comprehensive surgical resection aiming to remove the lesion. Interestingly, only one lesion was surgically excised, as the remaining lesions seem to be regressing spontaneously. The patient showed no signs of recurrence during the 12-month follow-up period.
Discussion
In pediatric patients, EH occurs more frequently in males with a median age of 14.4 years [7]. Among adult populations; however, the literature reports a higher prevalence in women with a median age ranging from 20–50 years old and a mean onset of 30–33 years old [8],[9],[10],[11],[12],[13],[14],[15],[16],[17],[18]. Our current case involves a 2-year-old Vietnamese patient diagnosed with EH. Epithelioid hemangioma is most prevalent among Asian and Caucasian populations, a trend that aligns with our case, and is considered rare in elderly and non-Asian pediatric populations [10],[15],[17],[18].
Epithelioid hemangioma is commonly seen in the head and neck region in both pediatric and adult populations [1],[2],[4],[6],[7],[8],[10],[14],[15],[16],[17],[18],[19],[20],[21],[22], aligning with our case presentation in the left frontal bone of the skull. However, the location of EH lesions can vary throughout the body [5],[7],[8],[10] (Table 1 and Table 2).
Additionally, our patient displayed symptoms of lesion growth and pain, mirroring symptoms reported in other pediatric EH cases [2],[7],[11]. However, the commonly reported symptoms among other cases include pruritus and pain [19]. Imaging revealed multiple ring-enhancing intra-axial lesions located predominantly in the gray-white matter junction of bilateral frontal left-sided parietal, left-sided occipital, left-sided temporal lobes surrounded by significant vasogenic edema. Also, the calvarium showed multiple lytic diploic lesions predominantly on the left side of the calvarium and with soft tissue components. A skeletal survey revealed no other lytic lesions. Abdominal ultrasound showed an enlarged liver with no focal lesion, normal spleen, and no lymphadenopathy. Interestingly within the adult literature, only one case has been reported in a 66-year-old woman with primary EH affecting the central nervous system (CNS) and manifesting in the CNS [22].
Regarding the pediatric population, there is no current treatment consensus for EH due to its rarity [7],[10]. Pediatric cases of EH [1],[2],[3],[4],[5],[10], including the presented case, typically present as well-circumscribed lesions, allowing for feasible complete surgical excision, a pattern similarly observed in most adult lesions [13],[14],[17],[19]. In our case, the lesion was dissected freely from the bone—falling in line with the most common method of treatment for EH reported in both pediatric and adult populations [1],[2],[4],[6],[7],[8],[10],[14],[15],[16],[17],[18],[19],[20]. Surgical excision typically results in low morbidity and has been deemed the most successful treatment [1],[2],[4],[6],[8],[10]. Similarly, the literature reports complete excision with follow-up as the most effective treatment of EH in adults [14],[15],[16],[17],[18],[19],[20],[21],[22].
The rarity of EH within the pediatric population has contributed to the difficulty in the characterization of diagnoses, as highlighted in previous literature [3],[4],[7],[10]. Similarly, the adult literature also indicates a lack of therapeutic protocols for EH in the brain due to its infrequent occurrence [22]. Despite these challenges, our case aligns with the treatment and outcome consensus observed in pediatric cases of EH. Successful outcomes and no recurrence are evident post-treatment and follow-up, mirroring findings in the literature [6]. While some cases, both in pediatric and adult populations, have reported medical treatments involving propranolol, sirolimus, and steroids, the necessity for long-term follow-up is emphasized to ensure successful outcomes in patients undergoing such interventions [7],[8],[14],[15],[16].
Our patient experienced numerous back-to-back viral infections following surgical treatment. However, he is doing well with no recurrence of EH during the 12-month follow-up.
Conclusion
We report a rare case of EH in a 2-year-old male. Notably, our patient’s very young age, multifocal lesion distribution, and unexpected outcome with lesion involution during follow-up distinguish this case. Additionally, the novelty of intracranial involvement expands the understanding of EH beyond its typical presentation limited to the skull and scalp.
REFERENCES
1.
Abrahim MJ, Gregory ND, Chennupati SK. Epithelioid hemangioma of the internal carotid artery: A case report supporting the reactive pathogenesis hypothesis of this vascular tumor. Int J Pediatr Otorhinolaryngol 2014;78(7):1186–9. [CrossRef]
[Pubmed]

2.
Avallone AN, Avallone MA, Share S, Rubin BP. Epithelioid hemangioma—A rare scrotal tumor of childhood. Urology 2012;80(3):707–9. [CrossRef]
[Pubmed]

3.
Chakravarthi SS, Veeraraghavan S, Fukui M, Jennings J, Rovin RA, Kassam AB. Diagnostic dilemma: An unusual case of primary intracranial, intraparenchymal epithelioid hemangioma. J Neurol Stroke 2018;8(5):263–5. [CrossRef]

4.
Floris G, Deraedt K, Samson I, Brys P, Sciot R. Epithelioid hemangioma of bone: A potentially metastasizing tumor? Int J Surg Pathol 2006;14(1):9–15. [CrossRef]
[Pubmed]

5.
Huang SC, Zhang L, Sung YS, et al. Frequent FOS gene rearrangements in epithelioid hemangioma: A molecular study of 58 cases with morphologic reappraisal. Am J Surg Pathol 2015;39(10):1313–21. [CrossRef]
[Pubmed]

6.
Kohli P, Wang Y, Baker Z, et al. Epithelioid hemangioma of the glans penis in an adolescent male: A case report. Urology 2022;170:189–92. [CrossRef]
[Pubmed]

7.
Liu KX, Duggan EM, Al-Ibraheemi A, Shaikh R, Adams DM. Characterization of long-term outcomes for pediatric patients with epithelioid hemangioma. Pediatr Blood Cancer 2019;66(1):e27451. [CrossRef]
[Pubmed]

8.
Moss HB, Sines DT, Blatt J, Dutton JJ, Proia AD. Epithelioid hemangioma responsive to oral propranolol. Ophthalmic Plast Reconstr Surg 2012;28(4):e88–90. [CrossRef]
[Pubmed]

9.
Murshed K, Farghaly H. Multifocal epithelioid hemangioma of the penis in a 4-year-old child: A case report. Am J Dermatopathol 2020;42(5):372–4. [CrossRef]
[Pubmed]

10.
Wiggins CJ, Dibbs RP, Bartlett EL, Ashton DJ, Maricevich RS. Atypical presentation and management of an epithelioid hemangioma: A case report and review of the literature. Ann Pediatr Surg 2020;16(1):53. [CrossRef]

11.
Bregman JA, Jordanov MI. Epithelioid hemangioma occurring in the radial styloid of a 17-year-old boy—An unusual presentation of an uncommon neoplasm. Clin Imaging 2014;38(6):899–902. [CrossRef]
[Pubmed]

12.
Okada E, Matsumoto M, Nishida M, et al. Epithelioid hemangioma of the thoracic spine: A case report and review of the literature. J Spinal Cord Med 2019;42(6):800–5. [CrossRef]
[Pubmed]

13.
Nangia R, Puri A, Gupta R, Bansal S, Negi A, Chauhan I. Epithelioid hemangioma of lingual alveolar mucosa: An immunohistochemical case report. Case Rep Med 2014;2014:436240. [CrossRef]
[Pubmed]

14.
Nair M, Aron M, Sharma MC. Angiolymphoid hyperplasia with eosinophilia (epithelioid hemangioma) of the breast: Report of a case. Surg Today 2000;30(8):747–9. [CrossRef]
[Pubmed]

15.
Lee SM, Khwarg SI, Ahn JH. Epithelioid hemangioma of the orbit treated with oral propranolol in adult. Can J Ophthalmol 2018;53(6):e244–6. [CrossRef]
[Pubmed]

16.
Hussain JA, Al Zamel H, Nawaz B. Angiolymphoid hyperplasia with eosinophilia (epithelioid hemangioma) of the external auditory canal, an unusual presentation in an adult female: A case report. J Surg Case Rep 2018;2018(7):rjy145. [CrossRef]
[Pubmed]

17.
Al-Muharraqi MA, Faqi MK, Uddin F, Ladak K, Darwish A. Angiolymphoid hyperplasia with eosinophilia (epithelioid hemangioma) of the face: An unusual presentation. Int J Surg Case Rep 2011;2(8):258–60. [CrossRef]
[Pubmed]

18.
Aggarwal A, Keluskar V. Epithelioid hemangioma (angiolymphoid hyperplasia with eosinophilia) in the oral mucosa. Indian J Dent Res 2012;23(2):271–4. [CrossRef]
[Pubmed]

19.
Makhoul M, Hourseau M, Nassereddine H, Hamad WA, Khoueir N. Epithelioid hemangioma of the nasal cavity with atypical features: A case report and review of the literature. SN Compr Clin Med 2023;5(1):235. [CrossRef]

20.
Ismail M, Damato S, Freeman A, Nigam R. Epithelioid hemangioma of the penis: Case report and review of literature. J Med Case Rep 2011;5:260. [CrossRef]
[Pubmed]

21.
Henriques ÁCG, Vidal MTA, Gurgel CA, Rocha SLD, Júnior BC, de Azevedo RA, dos Santos JN. Epithelioid hemangioma in the oral mucosa – A case report. Srp Arh Celok Lek 2016;144(9–10):535–40. [CrossRef]
[Pubmed]

22.
Covelli C, Parente P, Icolaro N, Dimitri LMC, Vigna B, Popolizio T, Graziano P. Primary epithelioid hemangioma of the central nervous system: A case report and review of the literature. J Neuropathol Exp Neurol 2021;80(7):717–9. [CrossRef]
[Pubmed]

23.
Malakan Rad E, Radmehr H, Vasei M, Rahimi Rastgoo B. Giant congenital right atrial epithelioid-capillary hemangioma with prolonged QT interval: Case report and practical surgical treatment strategy for primary cardiac tumors in children based on 25-year review of 299 cases. Echocardiography 2018;35(9):1471–81. [CrossRef]
[Pubmed]

SUPPORTING INFORMATION
Author Contributions
Ayah Rashwan - Conception of the work, Design of the work, Acquisition of data, Analysis of data, Drafting the work, Revising the work critically for important intellectual content, Final approval of the version to be published, Agree to be accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved.
Chanpreet Riarh - Revising the work critically for important intellectual content, Final approval of the version to be published, Agree to be accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved.
Lauren Tristani - Conception of the work, Design of the work, Acquisition of data, Analysis of data, Revising the work critically for important intellectual content, Final approval of the version to be published, Agree to be accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved.
Joseph de Nanassy - Revising the work critically for important intellectual content, Final approval of the version to be published, Agree to be accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved.
Raveena Ramphal - Revising the work critically for important intellectual content, Final approval of the version to be published, Agree to be accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved.
Nagwa Wilson - Acquisition of data, Revising the work critically for important intellectual content, Final approval of the version to be published, Agree to be accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved.
Guarantor of SubmissionThe corresponding author is the guarantor of submission.
Source of SupportNone
Consent StatementWritten informed consent was obtained from the patient for publication of this article.
Data AvailabilityAll relevant data are within the paper and its Supporting Information files.
Conflict of InterestAuthors declare no conflict of interest.
Copyright© 2025 Ayah Rashwan et al. This article is distributed under the terms of Creative Commons Attribution License which permits unrestricted use, distribution and reproduction in any medium provided the original author(s) and original publisher are properly credited. Please see the copyright policy on the journal website for more information.