|
Case Report
Primary splenic non-germinal center B-cell large lymphoma with associated granulomatous inflammation: A rare presentation
1 Department of Pathology, Mohammed V Military Training Hospital, Rabat, Morocco
2 Faculty of Medicine and Pharmacy of Rabat, Mohamed V University, 10100 Rabat, Morocco
3 Department of Radiology, Mohammed V Military Training Hospital, Rabat, Morocco
4 Department of Internal Medicine, Mohammed V Military Training Hospital, Rabat, Morocco
Address correspondence to:
Sara El Ghaffouli
Department of Pathology, Mohammed V Military Training Hospital, Rabat,
Morocco
Message to Corresponding Author
Article ID: 100089Z11SG2025
Access full text article on other devices

Access PDF of article on other devices

How to cite this article
El Ghaffouli S, El Graini S, El Bougrini Z, Chahdi H, Essaoudi MA, Oukabli M. Primary splenic nongerminal center B-cell large lymphoma with associated granulomatous inflammation: A rare presentation. J Case Rep Images Pathol 2025;11(1):26–29.ABSTRACT
Introduction: Granulomatous inflammation has been reported in association with both Hodgkin and non- Hodgkin lymphomas. Its presence alongside lymphoma is uncommon and may complicate the diagnostic process.
Case Report: We report the case of a 72-year-old male who presented to our hospital with weight loss, night sweats, and general deterioration. His medical history included urothelial carcinoma treated with Bacillus Calmette–Guérin (BCG) therapy in 2020. Clinical examination revealed splenomegaly extending to the flank. An excisional splenic biopsy confirmed the diagnosis of diffuse large B-cell lymphoma (DLBCL) with associated granulomatous inflammation.
Conclusion: This case illustrates an atypical presentation of DLBCL with granulomatous inflammation, emphasizing the need for careful histopathological assessment in such scenarios.
Keywords: Association, Granulomatous inflammation, Primary splenic DLBCL
Introduction
Splenic primary tumors, both benign and malignant, are extremely uncommon [1], fewer than 1% of NHLs and fewer than 2% of all lymphomas are primary malignant lymphoma of the spleen (PMLS) [2],[3]. Primary splenic DLBCL (PS-DLBCL) is an uncommon and poorly understood type of malignant lymphoma [4].
Chronic granulomatous inflammation can arise from multiple causes, such as infections, autoimmune disorders, and exposure to toxins [5]. It has also been linked to various malignancies, including solid tumors and lymphomas, with Hodgkin lymphoma (HL) being the most commonly reported, followed by different types of non-Hodgkin lymphoma (NHL) [6].
To our knowledge, reports of diffuse large B-cell lymphoma (DLBCL) in adults associated with chronic granulomatous inflammation are extremely limited [7]. We present a case of primary splenic diffuse large B-cell lymphoma accompanied by chronic granulomatous inflammation.
Case Report
A 72-year-old patient with a history of idiopathic Parkinson’s disease under treatment and high-grade urothelial carcinoma treated with BCG therapy since 2020 presented with a 4-month history of significant weight loss exceeding 12 kg over one year, accompanied by night sweats and general deterioration. Clinical examination revealed splenomegaly extending to the flank, suggesting systemic involvement.
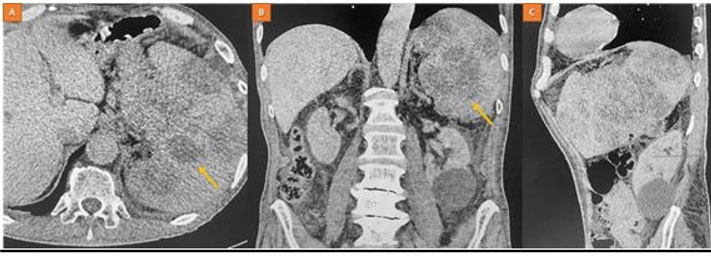
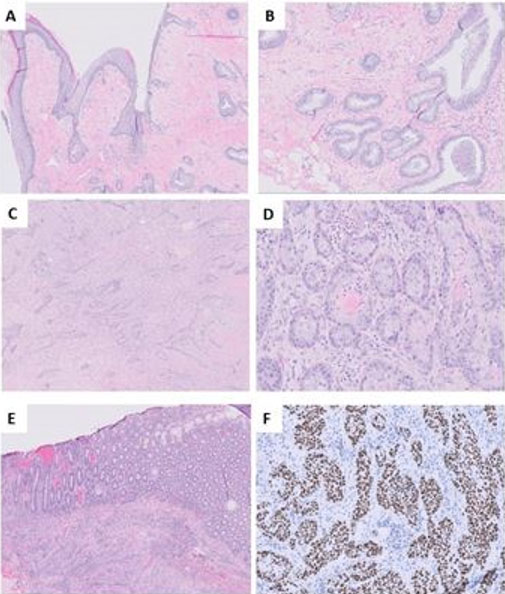
Biological findings demonstrated bicytopenia, characterized by non-regenerative normochromic normocytic anemia and lymphopenia at 700/mm³. Tumor markers and viral serologies, including human immunodeficiency virus (HIV), hepatitis, Epstein–Barr virus (EBV), and cytomegalovirus (CMV), were negative. An abdominal CT scan showed significant enlargement of the spleen with multiple hypodense, round-shaped splenic masses (Figure 1).
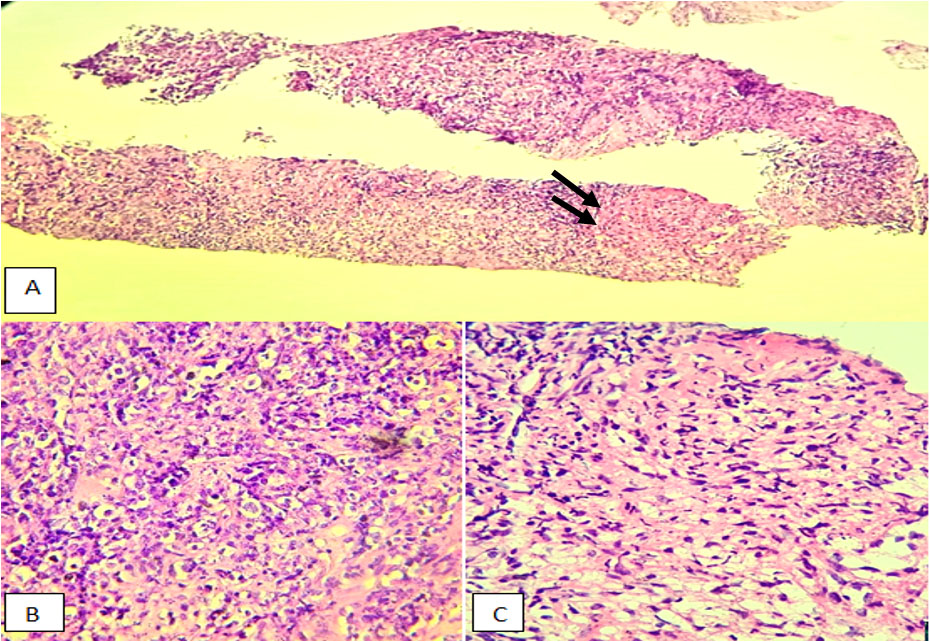
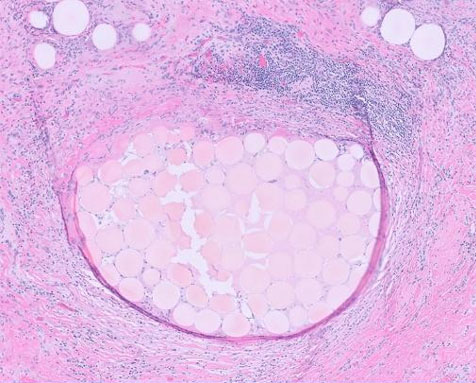
The histological study revealed a diffuse proliferation with a lymphoma-like appearance. The tissue consisted of large, non-cohesive tumor cells exhibiting marked anisokaryosis, vesicular chromatin, and prominent nucleoli. Mitotic figures were evident, and the cytoplasm appeared vacuolated (Figure 2).
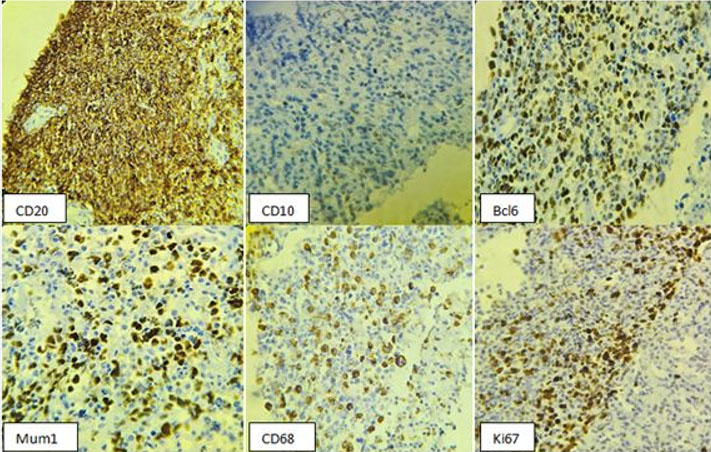
Immunohistochemical analysis demonstrated intense and diffuse staining of the tumor cells with the anti-CD20 antibody, confirming B-cell lineage. The anti-CD5 antibody marked reactive T lymphocytes. The anti-CD10 antibody was negative, while nuclear staining was positive for anti-Bcl2, anti-Bcl6, and anti-MUM1 antibodies. No staining was observed with the anti-CD68 antibody within the lymphomatous proliferation. The Ki67 proliferation index was estimated at 75%, indicating high proliferative activity. These findings supported the diagnosis of a diffuse large B-cell lymphoma (DLBCL), non-germinal center (non-GC) type, associated with granulomatous inflammation (Figure 3).
Discussion
Primary splenic diffuse large B-cell lymphoma is an uncommon condition that occurs less than 1% of the time. Splenomegaly, overall weakness, and soreness in the left upper quadrant are possible symptoms [1]. About one in three DLBCL patients also have fevers, sweats at night, and inexplicable weight loss [1]. Though it can happen at any age, it usually manifests in late middle life or later and affects males more often than women [8],[9].
Laboratory findings commonly reveal cytopenias, with anemia being the most frequent abnormality [10]. Primary splenic lymphoma (PSL) of diffuse large B-cell NHL showed up on imaging as splenomegaly accompanied by a distinct hypoechoicmass [11]. However sonographic differentiation between distinct subtypes of splenic lymphomas is not possible. A well-defined hypodense nodular mass inside the spleen may be visible on CT images [12].
Additionally, little is known about the process underlying the development of granulomas in non-Hodgkin lymphoma, especially B cell lymphoma [13]. Isolated case reports of exuberant granulomatous response in primary splenic NHL were found through a review of the literature. In 1977, Braylan et al. described three cases of primary splenic lymphoma with extensive granulomatous reaction [14]. This motivates additional investigation to elucidate its mechanism, therapeutic relevance, and consequences
Our patient had high-grade urothelial carcinoma treated with BCG therapy but it was not clear whether the granuloma formation is influenced by this treatment.
A splenectomy or core needle splenic biopsy is used to provide a conclusive diagnosis [15].
Histopathologically the World Health Organization classifies DLBCL as a tumor of medium to large B lymphoid cells with diffuse growth patterns. These cells’ nuclei are either more than twice as large as those of normal lymphocytes or are the same size as those of normal macrophages. Moreover, B-cell markers like CD19, CD20, CD22, CD79a, and PAX5 may be expressed by these cells [16],[17].
The hallmarks of chronic granulomatous inflammation include clusters of histiocytes (macrophages), which have granular eosinophilic cytoplasm with round-to-oval nuclei and an epithelioid appearance. These cells also combine to generate multinucleated giant cells [18].
The usual treatment for DLBCL is a splenectomy. Surgery can be combined with chemotherapy and radiation therapy to eradicate any malignant cells that may still be present. If the patient is not a surgical candidate, they can also be used alone; however, this is less effective. Palliative splenectomies may be used to treat patients with splenomegaly or cytopenia when there are metastases outside of the spleen [1].
A number of variables, including age, stage, performance status, extranodal involvement, and serum lactate dehydrogenase level, influence the prognosis in DLBCL [19]. Granulomas in HL or NHL have been suggested in a few papers to be linked to a better prognosis; however, it is still unknown and needs more research [20].
Conclusion
Primary splenic diffuse large B-cell lymphoma (DLBCL) with granulomatous inflammation is a rare and complex condition that requires a multidisciplinary diagnostic approach. The coexistence of granulomatous inflammation can complicate the clinical picture, often necessitating careful exclusion of infectious or autoimmune etiologies. Histopathological evaluation, immunohistochemistry, and molecular studies are crucial for accurate diagnosis.
REFERENCES
1.
VanVliet JL. Primary diffuse large B cell lymphoma of the spleen. Journal of Diagnostic Medical Sonography 2010;26(3):147–9. [CrossRef]

2.
Sun PG, Cheng B, Wang JF, He P. Fever of unknown origin revealed to be primary splenic lymphoma: A rare case report with review of the literature. Mol Clin Oncol 2017;6(2):177–81. [CrossRef]
[Pubmed]

3.
Shimizu-Kohno K, Kimura Y, Kiyasu J, et al. Malignant lymphoma of the spleen in Japan: A clinicopathological analysis of 115 cases. Pathol Int 2012;62(9):577–82. [CrossRef]
[Pubmed]

4.
Bairey O, Shvidel L, Perry C, et al. Characteristics of primary splenic diffuse large B-cell lymphoma and role of splenectomy in improving survival. Cancer 2015;121(17):2909–16. [CrossRef]
[Pubmed]

5.
Shah KK, Pritt BS, Alexander MP. Histopathologic review of granulomatous inflammation. J Clin Tuberc Other Mycobact Dis 2017;7:1–12. [CrossRef]
[Pubmed]

6.
Khurana KK, Stanley MW, Powers CN, Pitman MB. Aspiration cytology of malignant neoplasms associated with granulomas and granuloma-like features: Diagnostic dilemmas. Cancer 1998;84(2):84–91. [CrossRef]
[Pubmed]

7.
Nyunt WWT, Wong YP, Wan Jamaludin WF, Abdul Wahid SFS. Diffuse large B cell lymphoma with chronic granulomatous inflammation. Malays J Pathol 2016;38(1):55–9.
[Pubmed]

8.
Grosskreutz C, Troy K, Cuttner J. Primary splenic lymphoma: Report of 10 cases using the REAL classification. Cancer Invest 2002;20(5–6):749–53. [CrossRef]
[Pubmed]

9.
Lymphoma Research Foundation. Getting the Facts: Diffuse Large B-Cell Lymphoma. [Available at: https://www.lymphoma.org/]

10.
Ahmann DL, Kiely JM, Harrison EG Jr, Payne WS. Malignant lymphoma of the spleen. A review of 49 cases in which the diagnosis was made at splenectomy. Cancer 1966;19(4):461–9. [CrossRef]
[Pubmed]

11.
Ingle SB, Ingle CRH. Splenic lymphoma with massive splenomegaly: Case report with review of literature. World J Clin Cases 2014;2(9):478–81. [CrossRef]
[Pubmed]

12.
Khalid S, Daw JH, Daw H, Haddad A. Primary splenic diffuse large B-cell lymphoma: A rare case of massive splenomegaly and thrombocytopenia. Cureus 2018;10(7):e3026. [CrossRef]
[Pubmed]

13.
James DG. A clinicopathological classification of granulomatous disorders. Postgrad Med J 2000;76(898):457–65. [CrossRef]
[Pubmed]

14.
Braylan RC, Long JC, Jaffe ES, Greco FA, Orr SL, Berard CW. Malignant lymphoma obscured by concomitant extensive epithelioid granulomas: Report of three cases with similar clinicopathologic features. Cancer 1977;39(3):1146–55. [CrossRef]
[Pubmed]

15.
Pirzada S, Hasnain A, Mankani AA, Zahid I. Primary splenic diffuse large B-cell lymphoma: An atypical presentation. Cureus 2023;15(6):e40793. [CrossRef]
[Pubmed]

16.
17.
Swerdlow SH, Campo E, Pileri SA, et al. The 2016 revision of the World Health Organization classification of lymphoid neoplasms. Blood 2016;127(20):2375–90. [CrossRef]
[Pubmed]

18.
Shah KK, Pritt BS, Alexander MP. Histopathologic review of granulomatous inflammation. J Clin Tuberc Other Mycobact Dis 2017;7:1–12. [CrossRef]
[Pubmed]

19.
Haralambieva E, Rosati S, van Noesel C, et al. Florid granulomatous reaction in Epstein-Barr virus–positive nonendemic Burkitt lymphomas: Report of four cases. Am J Surg Pathol 2004;28(3):379–83. [CrossRef]
[Pubmed]

20.
Moran CA, Suster S, Przygodzki RM, Koss MN. Primary germ cell tumors of the mediastinum: II. Mediastinal seminomas—A clinicopathologic and immunohistochemical study of 120 cases. Cancer 1997;80(4):691–8. [CrossRef]
[Pubmed]

SUPPORTING INFORMATION
Author Contributions
Sara El Ghaffouli - Conception of the work, Design of the work, Acquisition of data, Analysis of data, Drafting the work, Revising the work critically for important intellectual content, Final approval of the version to be published, Agree to be accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved.
Soumya El Graini - Conception of the work, Design of the work, Drafting the work, Final approval of the version to be published, Agree to be accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved.
Zineb El Bougrini - Conception of the work, Design of the work, Drafting the work, Final approval of the version to be published, Agree to be accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved.
Hafsa Chahdi - Conception of the work, Design of the work, Drafting the work, Final approval of the version to be published, Agree to be accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved.
Mohamed Amine Essaoudi - Conception of the work, Design of the work, Drafting the work, Final approval of the version to be published, Agree to be accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved.
Mohamed Oukabli - Conception of the work, Design of the work, Drafting the work, Final approval of the version to be published, Agree to be accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved.
Guarantor of SubmissionThe corresponding author is the guarantor of submission.
Source of SupportNone
Consent StatementWritten informed consent was obtained from the patient for publication of this article.
Data AvailabilityAll relevant data are within the paper and its Supporting Information files.
Conflict of InterestAuthors declare no conflict of interest.
Copyright© 2025 Sara El Ghaffouli et al. This article is distributed under the terms of Creative Commons Attribution License which permits unrestricted use, distribution and reproduction in any medium provided the original author(s) and original publisher are properly credited. Please see the copyright policy on the journal website for more information.