|
Case Report
Bladder extra-gastrointestinal stromal tumor in an elderly patient in Uganda: A case report
1 Department of Pathology, Uganda Cancer Institute, Kampala, Uganda
2 Department of Pathology, Uganda Christian University, Mukono, Uganda
3 Department of Medical Oncology, Uganda Cancer Institute, Kampala, Uganda
Address correspondence to:
Tonny Okecha
Department of Pathology, Uganda Cancer Institute,
Uganda
Message to Corresponding Author
Article ID: 100090Z11TO2025
Access full text article on other devices

Access PDF of article on other devices

How to cite this article
Okecha T, Bakenga A, Waiswa A, Mawanda A, Nyakato V, Niyonzima N. Bladder extra-gastrointestinal stromal tumor in an elderly patient in Uganda: A case report. J Case Rep Images Pathol 2025;11(1):30–34.ABSTRACT
Introduction: Gastrointestinal stromal tumors (GISTs) are the most common mesenchymal neoplasms of the gastrointestinal tract (GI). They arise from the interstitial cells of Cajal or similar cells. Gastrointestinal stromal tumors that occur primarily outside the GI tract are termed extra-gastrointestinal stromal tumor (EGIST). These tumors are extremely rare and very few cases have been reported to date.
Case Report: We report a case of a 75-year-old male who presented with a bladder mass. The patient presented with hematuria and lower urinary tract symptoms of dribbling, straining and incomplete voiding of urine. Pelvic computed tomography scan showed a well-defined homogenous mass in the urinary bladder. The tumor was partially surgically resected and histology revealed a spindle-shaped tumor that was initially reported as fibrosarcoma. However, a histology review was done and immunohistochemistry staining was positive for CKIT, CD34, and DOG-1. A diagnosis of EGIST was made. There has not been any case of EGIST reported in Uganda to the best of our knowledge.
Conclusion: This case highlights the rarity of EGISTs, emphasizing the need for accurate diagnosis through immunohistochemistry and increased awareness for clinical management.
Keywords: Bladder, Extra-gastrointestinal, Stromal tumor, Uganda
Introduction
Gastrointestinal stromal tumors (GISTs) are some of the most common mesenchymal tumors of the gastrointestinal (GI) tract. Before the late 1990s, mesenchymal tumors arising in the GI tract were classified as smooth muscle tumors or neural tumors [1]. In 1983, Mazur and Clark introduced the term “stromal tumor” [2], but it was not widely accepted until the early 1990s, when CD34 was discovered as a marker for stromal tumors arising in the gastrointestinal tract, at that time being regarded as relatively specific. In addition, it was determined that a majority of tumors contained KIT mutations leading to constitutive activation of the molecule [3]. Since then, KIT-targeted therapy has significantly changed the management and prognosis of GIST patients.
Extra-gastrointestinal stromal tumors (EGISTs) are rare tumors with similar histopathological, molecular, and immunohistochemical characteristics as GIST but arise outside the GI tract. To the best of our knowledge, a few cases of EGIST from the bladder wall have been reported. Following a literature review, He and colleagues found 5 cases of patients with bladder EGISTs, including 3 men and 2 women, aged 34–78 years [4].
Case Report
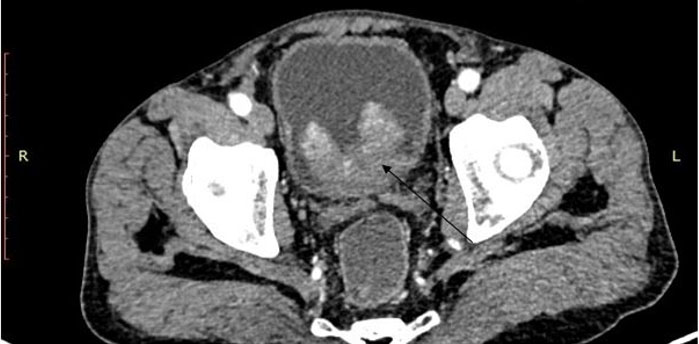
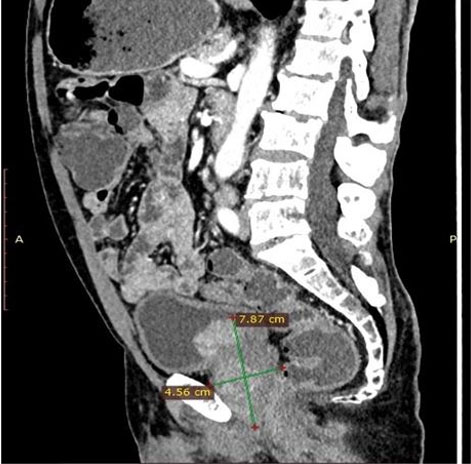
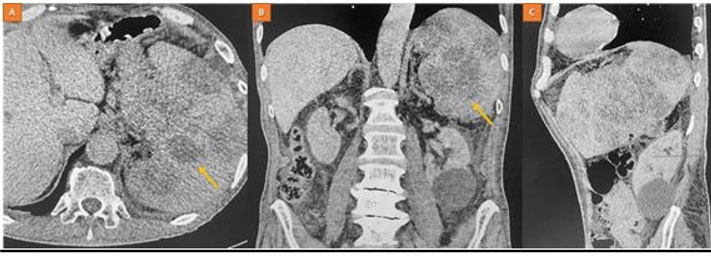
A 75-year-old male, African presented to the Uganda Cancer Institute (UCI), which is a tertiary cancer care facility in Uganda. The patient was a referral from a peripheral facility with a bladder mass, with a differential diagnosis of bladder cancer or prostate cancer given his age and presenting symptoms. Two months before referral, a partial transurethral resection of bladder tumor (TURBT) had been done, and the subsequent histology results were suggestive of fibrosarcoma, a diagnosis that was still queried by the primary clinician at the peripheral health facility. At UCI, the patient presented with a history of gross hematuria for one year, abdominal pain, dysuria, dribbling of urine, urgency and a feeling of incomplete voiding. A computed tomography scan of the pelvis done at UCI showed a residual tumor that was well-defined, and homogenous mass at the urinary bladder trigone, measuring 7.87 cm × 4.56 cm × 3.83 cm (Figure 1 and Figure 2). The mass appeared to originate from the posterior bladder wall and not extending beyond the bladder wall, no infiltration into the seminal vesicles, and no enlarged lymph nodes seen the gastrointestinal tract was free of any involvement by the mass as per the imaging findings.
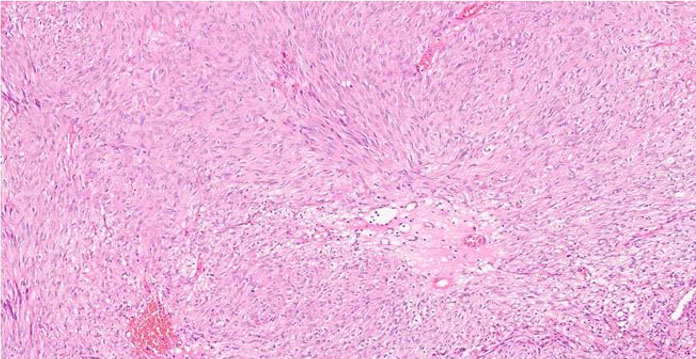
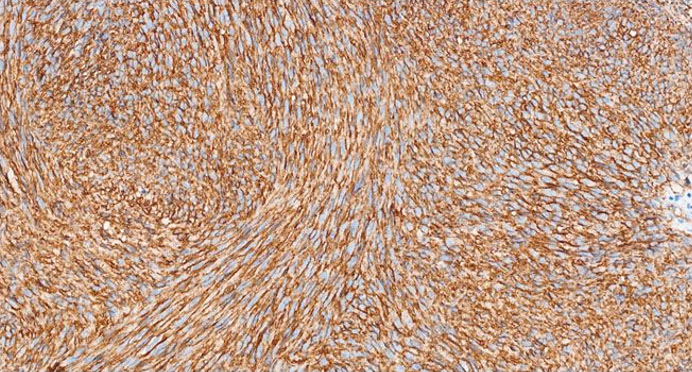
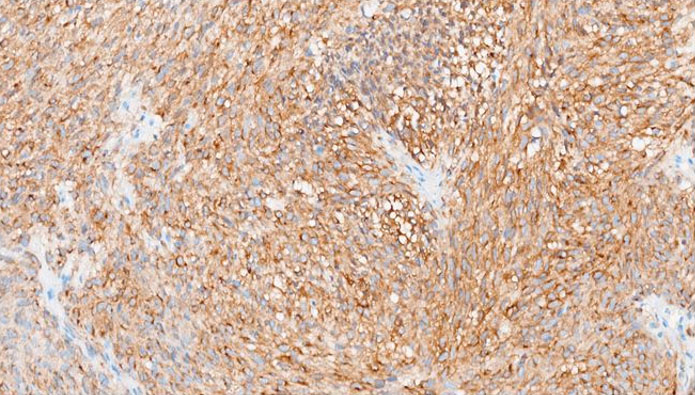
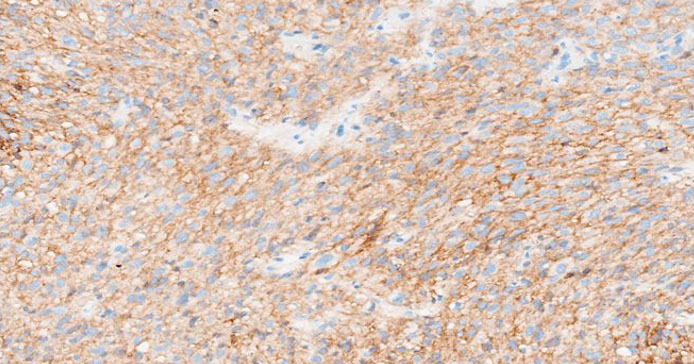
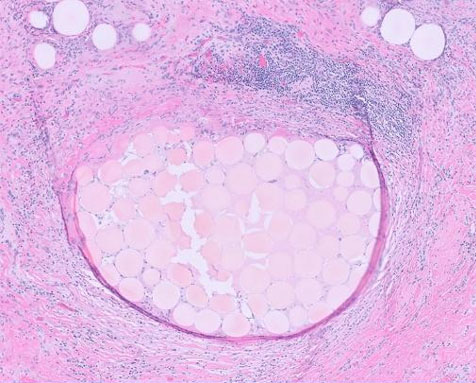
The formalin-fixed, paraffin-embedded (FFPE) tissue block from the previous biopsy was retrieved from the primary laboratory and then reviewed the histology at our facility, which revealed a tumor composed of spindle cells with fairly eosinophilic cytoplasm, inconspicuous nuclei, and rare abnormal mitoses (Figure 3). Immunohistochemically, the tumor cells were strongly positive for CD34 (Figure 4), CD117 (Figure 5), and DOG 1 (Figure 6).
A definitive diagnosis of EGIST was made and the patient was initiated on targeted therapy. Imatinib Mesylate 400 mg daily was prescribed for the patient, with regular Monthly reviews. After two months of treatment, there was marked symptomatic improvement as the hematuria and abdominal pain had ceased. However, during the 3rd-month review, it was noted that the patient had developed persistent pancytopenia [complete blood count (CBC) done, white blood cell (WBC) count = 1.8 × 103/µL, Neut = 0.80 × 103/µL, Hb = 8.4 g/dL, platelet (PLT) count = 80 × 103/µL), then treatment was halted. The patient was monitored for the next three months, was stable with no new symptom registered, CBC counts improved (WBC = 2.41 × 103/µL, Neut = 1.1 × 103/µL, Hb = 10.2 g/dL, PLT = 122 × 103/µL), and then Imatinib Mesylate was restarted at a reduced dose to 200 mg daily. A repeat of CT imaging after nine months of treatment also showed a marked reduction in the bladder tumor size to 4.25 cm × 3.93 cm × 3.05 cm. The patient was stable, with no new symptoms.
Discussion
Gastrointestinal stromal tumors are the most common mesenchymal tumors of the GI tract. They are most commonly present in the stomach (60%) and small intestine (25%), but they also arise in the rectum, colon, mesentery, ileum, and esophagus (15% together) [5]. Gastrointestinal stromal tumors are known to arise from the interstitial cells of Cajal since almost all GISTs express the KIT receptor tyrosine kinase which is evidently similar to the gastrointestinal Cajal cells whose role is to regulate the GI autonomic nerve system and peristalsis [6]. These cells also behave like stem cells which is demonstrated by their ability to transdifferentiate into smooth muscle [7]. Kitamura and colleagues reported that KIT-deficient mice lack gastrointestinal Cajal cells and those with introduced KIT-activating mutations develop Cajal cell hyperplasia and GISTs, which evidence supported the role of Cajal cells in GIST oncogenesis [8].
Before the advent of routine use of immunohistochemistry, the majority of GISTs were diagnosed as smooth muscle tumors or as tumors of the nerve sheath origin. Microscopically, GIST morphologies range from spindle cell to epithelioid, but are immunopositive for KIT (CD117) and/or DOG1 in essentially all cases.
Primary GISTs can rarely occur away from the GI tract with no connection whatsoever with the GI tract. When occurring away from the GI tract, they are referred to as extra-gastrointestinal stromal tumors (EGISTs). The incidence of EGISTs is reported to be 5–10% of all GISTs and approximately 80% are located in the Omentum or mesentery with the remainder developing in the retro peritoneum [9],[10]. Zhou et al. reported that the incidence of tumors in the mesentery was 50% (11/22), in the retroperitoneum was 36.4% (8/22)
Bladder extra-gastrointestinal stromal tumors are extremely rare with very few cases reported so far in the literature [4],[11],[12]. Moreover, most of these cases have been reported in Asian populations and not in Africa. A diagnosis of EGIST in the bladder requires a high index of suspicion by the evaluating pathologist and can easily be missed especially in the absence of immunohistochemistry (IHC) as is the case in many African pathology laboratories.
The tumor in our case report had a spindled morphology and was positive for CD34, CKIT, and DOG 1 immunostains.
CD34 is a transmembrane glycoprotein widely used as an immunohistochemical marker to identify hematopoietic stem cells, vascular endothelial cells, and stromal fibroblasts, as well as various neoplasms, spindle cell tumors (e.g., dermatofibrosarcoma protuberans, solitary fibrous tumors), and gastrointestinal stromal tumors (GISTs) [9],[13],[14]. Various studies emphasize its utility in diagnosing solitary fibrous tumors (SFTs) and its variable expression in GISTs, where it is positive in up to 83% of the cases [15]. CKIT is expressed in 80–95% of GISTs, showing cytoplasmic and membranous immunostaining with a characteristic perinuclear dot-like pattern [5]. CKIT IHC helps differentiate GISTs from other soft tissue neoplasms that may stain positive with cd34 on IHC [16]. Tyrosine kinase inhibitors (TKIs), such as Imatinib, Sunitib, Regorafenib, and Ripretinib, are first-line therapies for KIT-mutant GISTs. The commonly used drug has been Imatinib, demonstrating very excellent response rate in patients with KIT mutated GISTs [17]. Additional markers like DOG1 improve diagnostic accuracy, particularly in CKIT-negative GISTs [18]. DOG1 (Discovered On GIST 1), also known as ANO1, is a calcium-activated chloride channel protein that serves as a highly sensitive and specific immunohistochemical marker for gastrointestinal stromal tumors (GISTs). It is expressed in approximately 95% of GISTs, including those lacking KIT or PDGFRA mutations, making it particularly useful for diagnosing KIT-negative cases [18]. Beyond GISTs, DOG1 is also expressed in normal tissues such as interstitial cells of Cajal, salivary gland acini, prostate basal cells, and kidney distal tubules, as well as in tumors like esophageal squamous cell carcinoma, salivary gland tumors, chondroblastoma [19],[20],[21]. This therefore suggests that DOG1 is only linked to a diagnosis of GIST in spindle cell tumors, with numerous differential diagnoses in DOG1 positive epithelioid neoplasms.
Thus, immunoreactivity with both DOG1 and CKIT is confirmatory for GIST and/or EGIST since these immunostains have almost similar sensitivity of 94.4% and 94.7% respectively in GIST/EGIST [18]. Gastrointestinal stromal tumors and EGISTs have the same molecular profile, as well as similar histological and immunohistochemical behavior [22]. Treatment options for GISTs/EGISTs involve surgical resection for resectable tumors and use of tyrosine kinase inhibitors of which Imatinib Mesylate is the most commonly used agent even at our facility. The response to therapy is usually excellent in these patients underlining the importance of getting this diagnosis right.
Conclusion
Extra-gastrointestinal stromal tumors are exceptionally rare, and their diagnosis can be challenging due to their atypical presentation. This case underscores the importance of immunohistochemistry in differentiating EGISTs from other spindle cell tumors. Increased awareness and reporting of such cases is crucial for better understanding and management of this rare entity.
REFERENCES
1.
Fletcher CDM, Berman JJ, Corless C, et al. Diagnosis of gastrointestinal stromal tumors: A consensus approach. Hum Pathol 2002;33(5):459–65. [CrossRef]
[Pubmed]

2.
Mazur MT, Clark HB. Gastric stromal tumors. Reappraisal of histogenesis. Am J Surg Pathol 1983;7(6):507–19. [CrossRef]
[Pubmed]

3.
Hirota S, Isozaki K, Moriyama Y, et al. Gain-off-unction mutations of c-kit in human gastrointestinal stromal tumors. Science 1998;279(5350):577–80. [CrossRef]
[Pubmed]

4.
He F, Fang Z, Zhu P, Huang W, Li L. Bladder extragastrointestinal stromal tumor in an adolescent patient: A case-based review. Mol Clin Oncol 2014;2(6):960–2. [CrossRef]
[Pubmed]

5.
Miettinen M, Lasota J. Gastrointestinal stromal tumors (GISTs): Definition, occurrence, pathology, differential diagnosis and molecular genetics. Pol J Pathol 2003;54(1):3–24.
[Pubmed]

6.
Huizinga JD, Thuneberg L, Klüppel M, Malysz J, Mikkelsen HB, Bernstein A. W/kit gene required for interstitial cells of Cajal and for intestinal pacemaker activity. Nature 1995;373(6512):347–9. [CrossRef]
[Pubmed]

7.
Torihashi S, Nishi K, Tokutomi Y, Nishi T, Ward S, Sanders KM. Blockade of kit signaling induces transdifferentiation of interstitial cells of cajal to a smooth muscle phenotype. Gastroenterology 1999;117(1):140–8. [CrossRef]
[Pubmed]

8.
Kitamura Y, Hirotab S. Kit as a human oncogenic tyrosine kinase. Cell Mol Life Sci 2004;61(23):2924–31. [CrossRef]
[Pubmed]

9.
Miettinen M, Monihan JM, Sarlomo-Rikala M, et al. Gastrointestinal stromal tumors/smooth muscle tumors (GISTs) primary in the omentum and mesentery: Clinicopathologic and immunohistochemical study of 26 cases. Am J Surg Pathol 1999;23(9):1109–18. [CrossRef]
[Pubmed]

10.
Erlandson RA, Klimstra DS, Woodruff JM. Subclassification of gastrointestinal stromal tumors based on evaluation by electron microscopy and immunohistochemistry. Ultrastruct Pathol 1996;20(4):373–93. [CrossRef]
[Pubmed]

11.
Shin HS, Cho CH, Kum YS. Extragastrointestinal stromal tumor of the urinary bladder: A case report. Urol J 2011;8(2):165–7.
[Pubmed]

12.
Yu CH, Ting HK, Kao CC, Tsai WC, Wu ST, Yu DS. A rare case of extraluminal gastrointestinal stromal tumor of the ileum presenting with lower urinary tract symptoms: A case report. Medicine (Baltimore) 2019;98(49):e18103. [CrossRef]
[Pubmed]

13.
Nielsen JS, McNagny KM. Novel functions of the CD34 family. J Cell Sci 2008;121(Pt 22):3683–92. [CrossRef]
[Pubmed]

14.
Yang H, Yu L. Cutaneous and superficial soft tissue CD34+ spindle cell proliferation. Arch Pathol Lab Med 2017;141(8):1092–100. [CrossRef]
[Pubmed]

15.
van de Rijn M, Hendrickson MR, Rouse RV. CD34 expression by gastrointestinal tract stromal tumors. Hum Pathol 1994;25(8):766–71. [CrossRef]
[Pubmed]

16.
Sarlomo-Rikala M, Kovatich AJ, Barusevicius A, Miettinen M. CD117: A sensitive marker for gastrointestinal stromal tumors that is more specific than CD34. Mod Pathol 1998;11(8):728–34.
[Pubmed]

17.
18.
Miettinen M, Wang ZF, Lasota J. DOG1 antibody in the differential diagnosis of gastrointestinal stromal tumors: A study of 1840 cases. Am J Surg Pathol 2009;33(9):1401–8. [CrossRef]
[Pubmed]

19.
Akpalo H, Lange C, Zustin J. Discovered on gastrointestinal stromal tumour 1 (DOG1): A useful immunohistochemical marker for diagnosing chondroblastoma. Histopathology 2012;60(7):1099–106 [CrossRef]
[Pubmed]

20.
Khurram SA, Speight PM. Characterisation of DOG-1 expression in salivary gland tumours and comparison with myoepithelial markers. Head Neck Pathol 2019;13(2):140–8. [CrossRef]
[Pubmed]

21.
Jansen K, Farahi N, Büscheck F, et al. DOG1 expression is common in human tumors: A tissue microarray study on more than 15,000 tissue samples. Pathol Res Pract 2021;228:153663. [CrossRef]
[Pubmed]

22.
Sasmal PK, Sharma R, Patra S, Mishra TS, Mishra P, Rout B. Malignant extra-gastrointestinal stromal tumor of the mesentery. Surg J (N Y) 2019;5(3):e65–8. [CrossRef]
[Pubmed]

SUPPORTING INFORMATION
Author Contributions
Tonny Okecha - Conception of the work, Design of the work, Acquisition of data, Analysis of data, Drafting the work, Revising the work critically for important intellectual content, Final approval of the version to be published, Agree to be accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved.
Alex Bakenga - Acquisition of data, Revising the work critically for important intellectual content, Final approval of the version to be published, Agree to be accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved.
Ali Waiswa - Conception of the work, Design of the work, Drafting the work, Final approval of the version to be published, Agree to be accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved.
Anatoli Mawanda - Acquisition of data, Analysis of data, Drafting the work, Revising the work critically for important intellectual content, Final approval of the version to be published, Agree to be accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved.
Veronica Nyakato - Revising the work critically for important intellectual content, Final approval of the version to be published, Agree to be accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved.
Nixon Niyonzima - Acquisition of data, Revising the work critically for important intellectual content, Final approval of the version to be published, Agree to be accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved.
Guarantor of SubmissionThe corresponding author is the guarantor of submission.
Source of SupportNone
Consent StatementWritten informed consent was obtained from the patient for publication of this article.
Data AvailabilityAll relevant data are within the paper and its Supporting Information files.
Conflict of InterestAuthors declare no conflict of interest.