|
Case Report
ELOC-mutated renal cell carcinoma: “Unexpected occurrence in a younger adult”
1 Resident, Department of Pathology, The Ohio State University, Wexner Medical Center, Columbus, OH, USA
2 MD, PhD, Professor, Department of Pathology, The Ohio State University, Wexner Medical Center, Columbus, OH, USA
3 Chair Department of Pathology, The Ohio State University, Wexner Medical Center, Columbus, OH, USA
Address correspondence to:
Akansha Deshwal
Wexner Medical Center, Ohio State University, 410 W 10th Avenue, Columbus, Ohio,
USA
Message to Corresponding Author
Article ID: 100095Z11AD2025
Access full text article on other devices

Access PDF of article on other devices

How to cite this article
Deshwal A, Kumar H, Jones D, Parwani A. ELOC-mutated renal cell carcinoma: “Unexpected occurrence in a younger adult”. J Case Rep Images Pathol 2025;11(2):17–22.ABSTRACT
ELOC-mutated renal cell carcinoma (RCC) is a rare, molecularly defined RCC described in the 2022 WHO classification of genitourinary tumors. These tumors mostly affect middle-aged to elderly males, with approximately 30 cases reported in the English literature to date. A definitive diagnosis requires the identification of characteristic molecular alterations in conjunction with histopathological findings. Herein, we report an unusual case of ELOC-mutated RCC in a young male, identified by a mutation in the hotspot codon and a 1-copy loss of chromosome 8 sequences consistent with biallelic ELOC dysregulation. Due to its close resemblance to clear cell RCC, it is frequently misclassified, underscoring the importance of molecular characterization for accurate diagnosis. This case report aims to bridge the knowledge gap for this rare entity by focusing on clinical presentation, multimodal diagnostic approach, differential diagnosis, and prognostic considerations.
Keywords: Clear cell, ELOC-mutated RCC, TCEB-1
Introduction
The ever-evolving classification of kidney tumors has gained new recognition after the introduction of molecularly defined renal cell carcinomas (RCCs). Renal cell carcinomas with clear cell morphology showed a wide clinical spectrum, ranging from indolent tumors such as clear cell papillary RCC (CCPRCC) to aggressive subtypes like MITF-related RCC [1]. Within the spectrum of indolent tumors lies the rare ELOC-mutated RCC, formerly known as TCEB1-mutated RCC, which is recognized as a distinct entity in the WHO classification, 2022 [1],[2]. ELOC-mutated RCC accounts for 0.5% to 5% of clear cell RCC [3] and shares morphologic homology with RCC with leiomyomatous stroma (RCCLMS) [4], Von Hippel-Lindau (VHL)-mutated CCPRCC, and sporadic and hereditary TSC-associated RCC [5].
Among molecularly defined RCCs, ELOC-mutated RCCs are known as next-generation sequencing (NGS) identifiable tumors and exclusively recognized by the classic gene alteration, making it an essential diagnostic criterion [6]. These tumors were first described by Sato et al. in 2013, and subsequently, additional cases have been added [1]. However, to the best of our knowledge, fewer than 30 have been documented in elderly patients; its occurrence in younger individuals is exceedingly rare. Thus, we aim to present a case of incidental ELOC-mutated RCC in a young male, underscoring its clinicopathologic findings, histologic mimickers, and molecular insights into this rare and diagnostically challenging entity.
Case Report
A 35-year-old male patient was detected with an incidental renal mass during evaluation for an abnormal liver function test during his annual physical examination. A right upper quadrant ultrasound was performed, which showed a normal-sized right kidney, with a 2.5 × 2.3 × 1.9 cm solid mass within the lower pole demonstrating increased echogenicity relative to the adjacent cortex. The liver was mildly echogenic, reflecting a possible hepatic steatosis. A follow-up contrast-enhanced magnetic resonance imaging (MRI) was recommended for further evaluation of the renal mass. Magnetic resonance imaging urography revealed a mass arising from the lower pole of the right kidney laterally, with a heterogeneous T1 intermediate T2 signal mass measuring 2.8 × 2.3 × 2.1 cm, which showed a solid enhancement following contrast administration consistent with a renal cell neoplasm (Bosniak type IV). There were no additional lesions or hydronephrosis, and no perinephric edema. The right renal vein was patent.
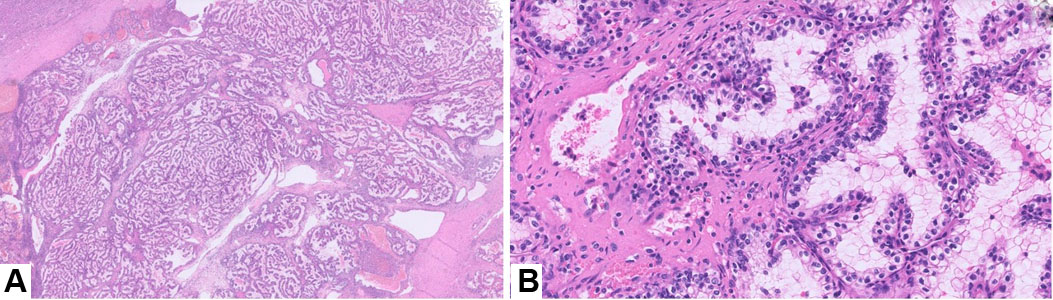
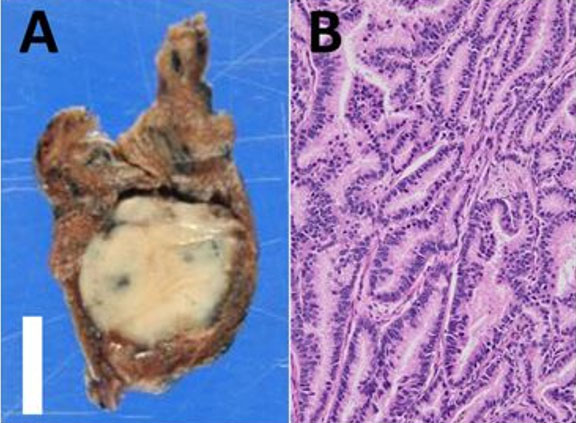
We received a partial nephrectomy specimen, which showed a tan-orange, soft, well-circumscribed nodule measuring 2.4 cm abutting the renal capsule. On histopathological examination, the tumor was encapsulated with tubulopapillary architecture. The lesion was characterized by thick, intersecting fibromuscular bands that gave a nodular growth pattern. The tumor cells had abundant clear cytoplasm, distinct cell borders, and hyperchromatic nuclei with irregular nuclear membranes (Figure 1).
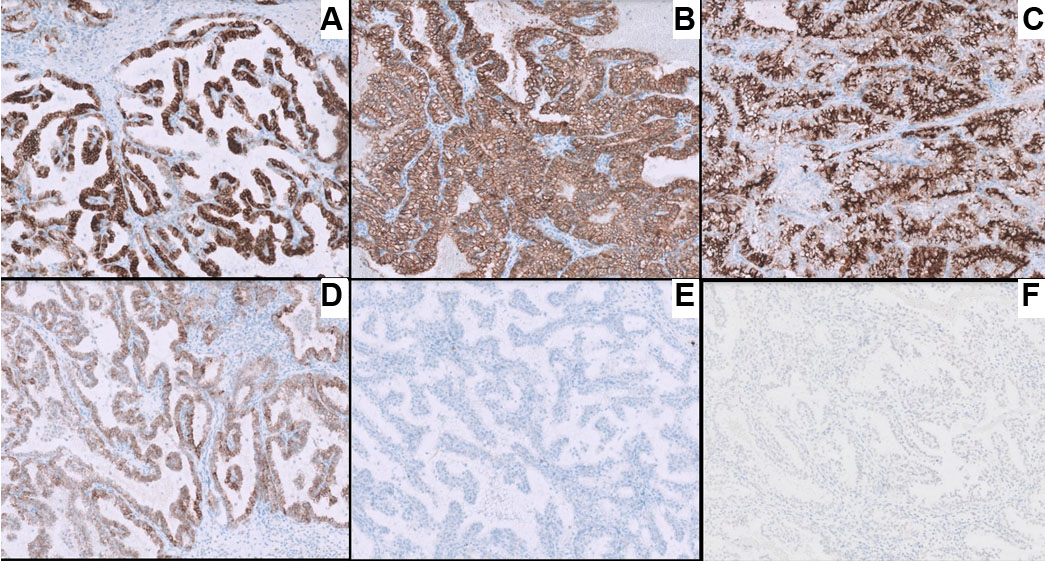
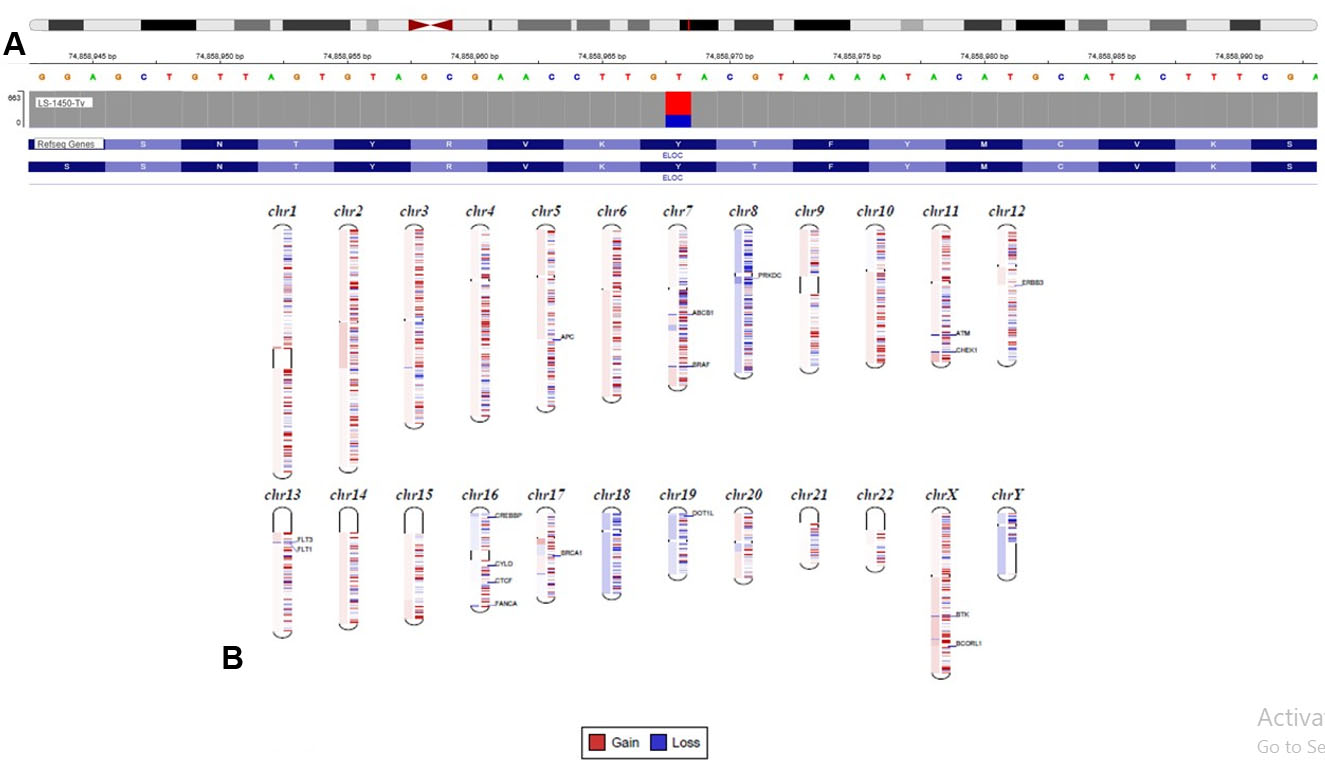
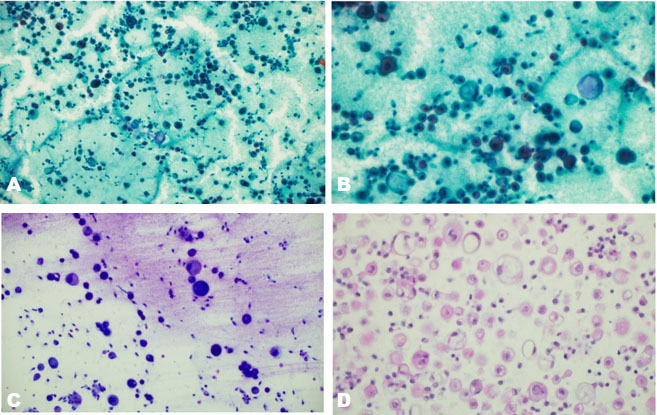
Immunohistochemistry showed tumor cell positivity for CD10, PAX8, alpha methylacyl CoA racemase (AMACR), AE1/AE3, and focal KRT7, with negativity for TFE3 and CD117 (Figure 2). Based on the above findings, a diagnosis of renal cell carcinoma with clear cell features was made. Next generation sequencing (NGS) was carried out using a 540-gene bait-probe-based assay on an Illumina 550 NextSeq with 300-cycle reagent kits (Illumina, San Diego, CA). The reagents used were IDT xGen gene capture probes (Integrated DNA Technologies, Inc., Coralville, IA) and KAPA HyperPrep Kit (Roche, Indianapolis, IN). Next generation sequencing detected a missense mutation at tyrosine 79 (Y79c), a hotspot codon of VHL-binding domain [7], representing a characteristic molecular signature mutation of ELOC-mutated RCC (Figure 3A). An EP300 inactivating variant was also detected, without mutations in VHL or other RCC-associated genes, and no TFE3 or TFEB fusions were identified [8]. Copy number analysis revealed loss of chromosome 8 sequences, including ELOC, without loss of the 3p chromosome (3p deletion is more characteristic of clear cell RCC, which helps differentiate from ELOC-mutated RCC). These observations were concordant with fluorescence in situ hybridization (FISH) results, which also did not show 3p deletion.
Discussion
Since the advent and implementation of molecular techniques, RCCs with clear cell change that appear morphologically similar have been recognized as molecularly defined entities, harboring driver mutations in TSC1, TSC2, MTOR, and VHL genes. More recently, RCC with ELOC mutations emerged as a molecularly defined RCC with characteristic histomorphology and molecular alterations. ELOC-mutated RCC was first reported by Sato et al. through molecular analysis of 106 clear cell RCCs, which identified 5 cases lacking the classic 3p deletion and/or VHL mutations. Instead, these tumors showed mutations in ELOC (formerly referred to as TCEB1) and/or showed deletion/loss of heterozygosity at the ELOC locus on chromosome 8 [1]. Since this initial discovery, a wider range of ELOC alterations has been described.
The ELOC gene, located at chromosome 8q21, encodes Elongin C, a subunit of the transcription factor B (SIII) complex and a component of the VHL complex. When mutated, the ELOC protein alters the association of HIF (hypoxia-inducing factor B) with the VHL complex, preventing its degradation by ubiquitination [6]. The stabilized HIF translocate into the nucleus, resulting in the overexpression of HIF target genes involved in dedifferentiation, VEGF production, and cell migration, facilitating tumorigenesis as well as metastatic processes [8]. Molecularly, these tumors harbor unique genetic changes in the ELOC mutation sites at the pVHL binding domain: A100, I95, and Y79 [9]. In our case, a Y79C mutation (and an EP300 inactivating variant) with no loss of chromosome 3p was identified, concurrent with previous studies suggesting that these aberrations are mutually exclusive [10].
ELOC-mutated RCC is an extremely rare entity. However, the limited literature suggests that it typically occurs in middle-aged and elderly male patients, with a male-to-female ratio of 22:1. The usual age of presentation is 38–77 years, with a majority of patients aged approximately 50 years old, as compared to translocation-related RCCs, and may present incidentally [4],[11]. In our case, the tumor was detected incidentally at a relatively early age compared to previous literature.
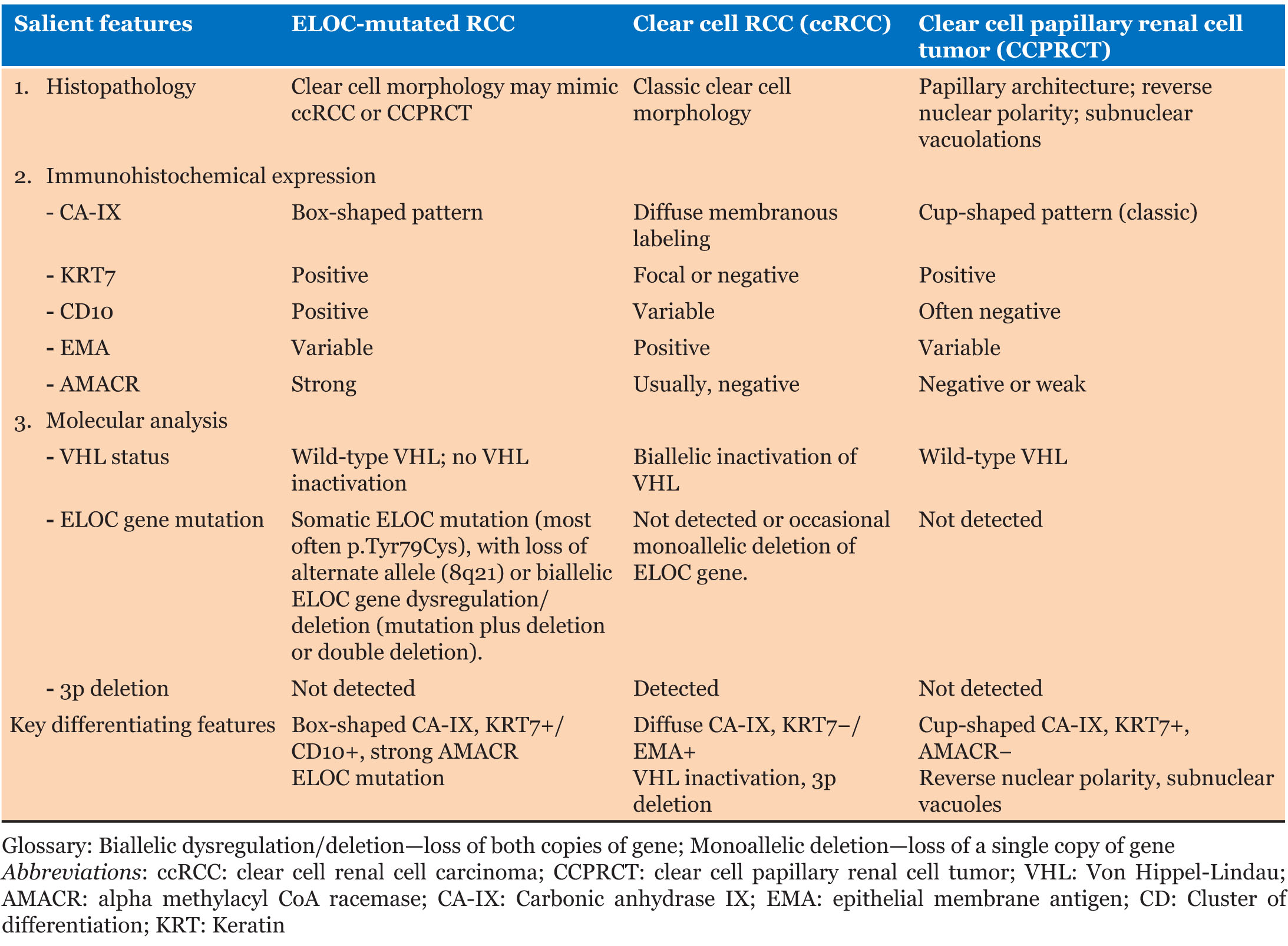
Histopathologically, the tumor shows predominance of clear cells with voluminous cytoplasm with unique histological findings, such as smooth muscle predominant fibrous pseudocapsule and fibromuscular bands more prominent at the periphery of the tumor, giving the tumor nodular to branched acinous or tubular architecture, and infrequently, focal papillary formations may be noted [4],[12]. However, due to clear cell morphology, these tumors share morphological homology with existing and novel entities like clear cell renal cell carcinoma (ccRCC), RCCs with fibromuscular stroma (RCCFMS), and clear cell papillary renal cell tumor (CCPRCT) [10] and are unanimously differentiated by subtle histopathological findings and two distinguished immunohistochemical stains, i.e., CAIX and KRT7. ELOC-mutated RCC bears a distinct immunohistochemical (IHC) profile characterized by a combination of clear-cell and non-clear-cell markers− box-shaped carbonic anhydrase IX labeling (CA IX), CD10+/KRT7+, AMACR+, epithelial membrane antigen (EMA+/–) (Figure 2, Table 1) [11]. In contrast, ccRCC exhibits diffuse membranous CAIX labeling, KRT7 focal/−, while CCPRCT shows cup shaped CAIX labeling, KRT7+, CD10−, EMA+/−, AMACR−, and reverse nuclear polarity with subnuclear vacuolation (Table 1) [12]. The fibromyomatous stroma seen in ELOC-mutated RCC often shows positivity for SMA and desmin.
At molecular level, ELOC-mutated RCC display distinct wild-type VHL and somatic mutations in the TCEB1 gene, as well as loss of the alternate allele (8q21) [1], which contrasts with the biallelic inactivation of the VHL gene found in ccRCC (Table 1) [13].
Nevertheless, molecular characterization of ELOC mutation is of paramount importance in histologically indistinguishable RCCFMS caused by mutations of the mTOR pathway [4],[6],[14], but before the molecular era, the analogous tumors were blanketed under the same category [15]. However, in previous studies, biallelic inactivation of ELOC and TSC/MTOR mutations were found to be mutually exclusive of each other, supporting the notion that RCCFMS and ELOC-mutated RCCs are distinct entities [10].
Thus, a definitive diagnosis of ELOC-mutated RCC can exclusively be determined by complex molecular analysis [10], i.e., absence of 3p deletion and/or VHL mutations and biallelic ELOC deletion (loss of both copies of the ELOC gene), while monoallelic deletion (loss of a single copy of the ELOC gene) with clear cell morphology should be diagnosed as ccRCC [10]. However, by convention, renal tumors with clear cell morphology in the absence of molecular confirmation can be designated as “KRT7 positive ccRCCs with prominent fibromuscular septations,” and differentials may be suggested [16].
However, even in optimally and radically resected tumors, accurately recognizing this entity could avert unnecessary adjuvant therapies typically used for ccRCC [6]. Unlike metastatic ccRCC, which often requires VEGFR tyrosine kinase inhibitors (TKIs), mTOR inhibitors, or immune checkpoint inhibitors, ELOC-mutated RCC is generally indolent, and surgery alone is usually sufficient for localized disease [17]. Molecularly, Hakimi et al. found that ELOC-mutated RCCs lack mutations in PBRM1, SETD2, and BAP1, as seen in more aggressive, VHL-inactivated RCCs [11].
Although ELOC-mutated RCCs are rare variants, they are known to have indolent behavior and are not clinically challenging, as the surgical resection is often curative. In the recent largest institutional series of 1209 RCCs, ELOC-mutated RCC (11/13) demonstrated a favorable overall and progression-free survival as compared to the stage and grade-matched VHL-null clear cell RCC. However, occasional ELOC-RCC (2/13) with solid alveolar pattern, necrosis, copy number variations, and extra monoallelic VHL copy loss correlated with high-stage and aggressive behavior [18]. A similar observation was made by diNatale et al., in which most of the tumors (4/5) were staged higher due to the presence of high-grade features like pleomorphism, prominent nucleoli, and necrosis, and clinically also found to have distant metastasis [8]. Although, due to rarity, there is no consensus on follow-up guidelines for ELOC-mutated RCC, the presence of high-grade, aggressive tumors in the literature underscores the importance of long-term surveillance even when low risk is anticipated [19].
Thus, heterogeneous clinical spectrum, limited available literature, lack of follow-up guidelines, and gap in molecular targeted therapies for ELOC-mutated RCC underscores the importance of larger pooled case series and evidence-based studies to unfold the clinical outcome and biological behavior of this rare entity to guide future targeted therapies.
Conclusion
ELOC-mutated RCC should be considered even in young age groups, as it differs considerably from ccRCC in clinical behavior, prognosis, and treatment strategies. For renal tumors with clear cell morphology—a combined multimodal diagnostic approach including histopathology, IHC, and molecular analysis should be employed for accurate disease characterization. Importantly, the presence of the signature ELOC mutation remains the essential criterion for diagnosis.
REFERENCES
1.
Sato Y, Yoshizato T, Shiraishi Y, Maekawa S, Okuno Y, Kamura T, et al. Integrated molecular analysis of clearcell renal cell carcinoma. Nat Genet 2013;45(8):860–7. [CrossRef]
[Pubmed]

2.
3.
Hu X, Tan C, Zhu G. Clinical characteristics of molecularly defined renal cell carcinomas. Curr Issues Mol Biol 2023;45(6):4763–77. [CrossRef]
[Pubmed]

4.
Wang Y, Zhao P, Wang L, Wang J, Ji X, Li Y, et al. Analysis of clinicopathological and molecular features of ELOC (TCEB1)-mutant renal cell carcinoma. Pathol Res Pract 2022;235:153960. [CrossRef]
[Pubmed]

5.
Shah RB, Stohr BA, Tu ZJ, Gao Y, Przybycin CG, Nguyen J, Cox RM, et al. “Renal cell carcinoma with leiomyomatous stroma” harbor somatic mutations of TSC1, TSC2, MTOR, and/or ELOC (TCEB1): Clinicopathologic and molecular characterization of 18 sporadic tumors supports a distinct entity. Am J Surg Pathol 2020;44(5):571–81. [CrossRef]
[Pubmed]

6.
Rizzo M, Caliò A, Brunelli M, Pezzicoli G, Ganini C, Martignoni G, et al. Clinico-pathological implications of the 2022 WHO renal cell carcinoma classification. Cancer Treat Rev 2023;116:102558. [CrossRef]
[Pubmed]

7.
Pan X, Tu H, Mohamed N, Avenarius M, Caruthers S, Zhao W, et al. Find DNA fusion: An analytical pipeline with multiple software tools improves detection of cancer-associated gene fusions from genomic DNA. J Mol Diagn 2024;26(2):140–9. [CrossRef]
[Pubmed]

8.
DiNatale RG, Gorelick AN, Makarov V, Blum KA, Silagy AW, Freeman B, et al. Putative drivers of aggressiveness in ELOC (TCEB1)-mutant renal cell carcinoma: An emerging entity with variable clinical course. Eur Urol Focus 2021;7(2):381–9. [CrossRef]
[Pubmed]

9.
Chen XQ, Huang J, Lan Y, Wu YL, Yan XC, Bian XW, et al. ELOC-mutated renal cell carcinoma with new mutation site combined with lung adenocarcinoma: Report of a case. [Article in Chinese]. Zhonghua Bing Li Xue Za Zhi 2024;53(8):861–63. [CrossRef]
[Pubmed]

10.
Batavia AA, Rutishauser D, Sobottka B, Schraml P, Beerenwinkel N, Moch H. Biallelic ELOC-inactivated renal cell carcinoma: Molecular features supporting classification as a distinct entity. Mod Pathol 2023;36(8):100194. [CrossRef]
[Pubmed]

11.
Hakimi AA, Tickoo SK, Jacobsen A, Sarungbam J, Sfakianos JP, Sato Y, et al. TCEB1-mutated renal cell carcinoma: A distinct genomic and morphological subtype. Mod Pathol 2015;28(6):845–53. [CrossRef]
[Pubmed]

12.
Li H, Argani P, Halper-Stromberg E, Lotan TL, Merino MJ, Reuter VE, et al. Positive GPNMB immunostaining differentiates renal cell carcinoma with fibromyomatous stroma associated with TSC1/2/MTOR alterations from others. Am J Surg Pathol 2023;47(11):1267–73. [CrossRef]
[Pubmed]

13.
Cancer Genome Atlas Research Network. Comprehensive molecular characterization of clear cell renal cell carcinoma. Nature 2013;499(7456):43–9. [CrossRef]
[Pubmed]

14.
Alaghehbandan R, Siadat F, Trpkov K. What’s new in the WHO 2022 classification of kidney tumours? Pathologica 2022;115(1):8–22. [CrossRef]
[Pubmed]

15.
Trpkov K, Hes O. New and emerging renal entities: A perspective post-WHO 2016 classification. Histopathology 2019;74(1):31–59. [CrossRef]
[Pubmed]

16.
17.
Pezzicoli G, Ciciriello F, Musci V, Salonne F, Ragno A, Rizzo M. Genomic profiling and molecular characterization of clear cell renal cell carcinoma. Curr Oncol 2023;30(10):9276–90. [CrossRef]
[Pubmed]

18.
Wang JJ, Huang RR, Cone BD, Kang SH, Setoodeh R, Sisk AE, et al. ELOC-mutated renal cell carcinoma is a rare indolent tumor with distinctive genomic characteristics. Mod Pathol 2025;38(8):100777. [CrossRef]
[Pubmed]

19.
Millan B, Loebach L, Blachman-Braun R, Patel MH, Saini J, Linehan WM, et al. Molecular genetics of renal cell carcinoma: A narrative review focused on clinical relevance. Curr Oncol 2025;32(6):359. [CrossRef]
[Pubmed]

SUPPORTING INFORMATION
Author Contributions
Akansha Deshwal - Conception of the work, Design of the work, Acquisition of data, Analysis of data, Drafting the work, Revising the work critically for important intellectual content, Final approval of the version to be published, Agree to be accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved.
Himani Kumar - Revising the work critically for important intellectual content, Final approval of the version to be published, Agree to be accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved.
Daniel Jones - Conception of the work, Design of the work, Drafting the work, Revising the work critically for important intellectual content, Final approval of the version to be published, Agree to be accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved.
Anil Parwani - Conception of the work, Design of the work, Revising the work critically for important intellectual content, Final approval of the version to be published, Agree to be accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved.
Guarantor of SubmissionThe corresponding author is the guarantor of submission.
Source of SupportNone
Consent StatementWritten informed consent was obtained from the patient for publication of this article.
Data AvailabilityAll relevant data are within the paper and its Supporting Information files.
Conflict of InterestAuthors declare no conflict of interest.
Copyright© 2025 Akansha Deshwal et al. This article is distributed under the terms of Creative Commons Attribution License which permits unrestricted use, distribution and reproduction in any medium provided the original author(s) and original publisher are properly credited. Please see the copyright policy on the journal website for more information.