|
Case Report
Massive hemorrhage and osteonecrosis of the jaw in the patient with methotrexate-associated lymphoproliferative disorder: Report of a case and review of literature
1 Department of Dentistry and Oral Surgery, Kawasaki Municipal Kawasaki Hospital, Kawasaki, Japan
2 Department of Dentistry and Oral Surgery, School of Medicine, Keio University, Tokyo, Japan
3 Department of Dentistry and Oral Surgery, Kawasaki Municipal Ida Hospital, Kawasaki, Japan
Address correspondence to:
Takazumi Yasui
DDS, PhD, Department of Dentistry and Oral Surgery, Kawasaki Municipal Kawasaki Hospital, 12-1 Shinkawadori, Kawasaki-ku, Kawasaki, Kanagawa 210-0013,
Japan
Message to Corresponding Author
Article ID: 101202Z01MK2021
Access full text article on other devices

Access PDF of article on other devices

How to cite this article
Kimura M, Yasui T, Nagamine H, Yajima S, Kodaka R, Onizawa K. Massive hemorrhage and osteonecrosis of the jaw in the patient with methotrexate-associated lymphoproliferative disorder: Report of a case and review of literature. Int J Case Rep Images 2021;12:101202Z01MK2021.ABSTRACT
Introduction: Methotrexate-associated lymphopro-liferative disorder (MTX-LPD) is one of the adverse effects of methotrexate (MTX). We report a case of MTX-LPD that caused osteonecrosis and massive bleeding without regression following MTX withdrawal.
Case Report: A 61-year-old woman was referred to our hospital because of delayed socket healing after dental extraction. She had been diagnosed with rheumatoid arthritis (RA) and received MTX treatment for six years. At the first visit, a 15 mm × 15 mm bone exposure and swelling of the surrounding gingiva in the right upper molar region were observed. We performed an incisional biopsy of the necrotic bone and surrounding mucosal tissue. The histopathological diagnosis was diffuse large B-cell lymphoma (DLBCL). Therefore, we referred the patient to the Department of Hematology in our hospital. She was diagnosed with MTX-LPD based on the history of MTX therapy for RA, and MTX was discontinued immediately. However, the lesion progressed and caused bleeding that required blood transfusion and hemostasis by microfibrous collagen and tie-over even after the withdrawal of MTX. Subsequently, rituximab, cyclophosphamide, vincristine, and prednisolone (R-COP) chemotherapy was initiated. After 3 courses of chemotherapy, sequestrectomy was performed under local anesthesia. She achieved complete remission after 8 courses of chemotherapy, and there was no recurrence of necrotic bone exposure or gingival swelling.
Conclusion: Approximately 80% of MTX-LPD cases occurring in the oral region show regression after MTX discontinuation, but we experienced a case in which bleeding occurred with progression of the lesion after discontinuation. Careful follow-up is required during the MTX discontinuation period.
Keywords: Diffuse large B-cell lymphoma, Massive hemorrhage, Methotrexate-associated lymphoproliferative disorder, Osteonecrosis of the jaw
Introduction
Methotrexate (MTX) is currently the most widely used first-line conventional synthetic disease-modifying anti-rheumatic drug (csDMARD) in the treatment of rheumatoid arthritis (RA) [1]. It has been associated with a range of adverse effects, including cytopenia, serious infections, liver damage, synovitis, myelopathy, interstitial pneumonia, mucocutaneous ulcer, and MTX-associated lymphoproliferative disorder (MTX-LPD). Methotrexate-associated lymphoproliferative disorder, which is classified as other iatrogenic immunodeficiency-associated LPD in the WHO classification 2017, rarely occurs in the oral and maxillofacial region [2]. Medication-related osteonecrosis of the jaw (MRONJ), which is associated with bisphosphonates, anti-receptor activator of nuclear factor κB ligand (anti-RANKL) (denosumab), and antiangiogenic medications, has been widely recognized [3]. Recently, it has been reported that several cases of MTX-LPD in the gingiva accompany osteonecrosis of the jaw (ONJ) without antiresorptive and antiangiogenic medications [4],[5],[6]. Besides, to our knowledge, half of the MTX-LPD cases show regression by MTX withdrawal [2],[7], and no case that caused massive bleeding because of progression of the lesion has been reported in the oral region. Here, we report a case of MTX-LPD with jaw osteonecrosis and massive hemorrhage as a result of progression of the lesion after MTX withdrawal. In addition, we performed a systematic literature review to investigate cases of MTX-LPD in the oral region.
Case Report
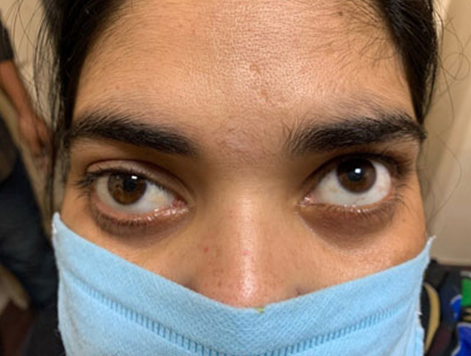
A 61-year-old woman was treated with extraction of the maxillary right first and second molars at dental clinic in August 2017. One month later, the patient was referred to Department of Dentistry and Oral Surgery, because of delayed socket healing after dental extraction. Her past medical history was significant for RA and myocardial infarction. The RA had been treated with MTX 12 mg once weekly since 2011. She had been taking cilostazol for the previous episode of myocardial infarction. There was no history of the use of other antirheumatic drugs, corticosteroids, antiresorptive, and antiangiogenic medications. At the first visit, oral examination revealed a 15 mm × 15 mm area of exposed bone with necrotic soft tissue in the right posterior maxilla (Figure 1). A reddish swelling of the gingiva was found in the surrounding exposed bone, but there was no bleeding or pain. A dental panoramic radiograph revealed absence of healing of the extraction sockets and osteosclerosis around the right upper molar. Computed tomography (CT) showed thickening of the maxillary sinus mucosa, while there was no obvious separation of the sequestrum (Figure 2A and Figure 2B). Abnormal fluorodeoxyglucose uptake (maximum standardized uptake value, 13.8) in the right posterior maxilla area was observed on positron emission tomography/CT (PET/CT) (Figure 2C). Furthermore, uptake by other organs, including the adrenal gland, multiple intrapulmonary nodules, muscles, bones, and lymph nodes was observed (Figure 2D). Blood tests revealed elevated levels of C-reactive protein (CRP, 2.01 mg/dL), soluble interleukin-2 receptor (sIL-2R, 1980 U/μL), and lactate dehydrogenase (LDH, 431 U/L), respectively. We considered the possibility of MTX-LPD and other malignant tumors, and performed an incisional biopsy in the exposed bone and surrounding mucosal tissue. Histopathological findings of the bone confirmed a sequestrum with empty osteocytic lacunae and bacterial infection, including colonies of Actinomyces spp. in the marrow space. It did not reveal tumor cells, including atypical lymphocytes, usually found in MTXL-PD. Pathological analysis of the mucosal tissue showed infiltration of numerous lymphocytes and diffuse proliferation of large-sized atypical lymphoid cells under the epithelium surrounding the gingiva (Figure 3A). Immunohistochemical analysis showed CD20+, CD3−,CD5−, and CD10− cells. Ki67+ was detected in 80% of these cells. Additionally, Epstein–Barr encoding region (EBER) was detected in almost all atypical cells by in situ hybridization (Figure 3B). Based on these findings, the histopathological diagnosis was diffuse large B-cell lymphoma (DLBCL) with jaw osteonecrosis. Therefore, we referred the patient to the Department of Hematology in our hospital. She was diagnosed with MTX-LPD based on the history of MTX therapy for RA, and MTX was discontinued immediately.
However, the lesion progressed and caused massive bleeding three weeks after the withdrawal of MTX. Therefore, she visited the emergency department of our hospital. Although the patient was conscious, a gait disorder due to dizziness was observed. Since vigorous bleeding was observed around the lesion, she was admitted to our department (Figure 4). Bleeding equivalent to class III as per the Advanced Trauma Life Support (ATLS) classification was observed [8]. The pterygoid venous plexus and posterior superior alveolar artery were considered as the blood vessels causing bleeding. Hemostasis was performed using a microfibrous collagen hemostatic material and tie-over under local anesthesia. Two units of concentrated red blood cells (RCC) were transfused since the blood tests revealed low hemoglobin (Hb) level (Hb: 7.8 g/dL). The next day, her Hb level further reduced to 6.3 g/dL, and transfusion of 2 units of RCC was added. Three days after admission, she was transferred to the Department of Hematology, and rituximab, cyclophosphamide, vincristine, and prednisolone (100 mg/day) (R-COP) chemotherapy was initiated. Doxorubicin was not used because of myocardial infarction history and cardiotoxicity. After 3 courses of chemotherapy, the separated sequestrum (34 × 23 × 18 mm size) in the right posterior maxilla was removed under local anesthesia (Figure 5). After 8 courses of chemotherapy, she achieved complete remission. Two years later, there was no recurrence of MTX-LPD, and her RA remained well controlled without MTX. Anemia was not observed (hemoglobin level 13 g/dL). Moreover, there was no recurrence of necrotic bone exposure or gingival swelling.
Discussion
In 1991, Ellman et al. first reported a lymphoproliferative disorder in patients with RA receiving MTX, and MTX-LPD was recognized as one of the side effects [9]. Although extranodal lesions have been reported in 40% of MTX-LPD cases, cases of the oral region are relatively rare. After performing a comprehensive English literature search, it was noted that 26 cases of MTX-LPD in the oral region have been reported, and 13 of them were accompanied by ONJ (Table 1) [4],[5],[6],[10],[11],[12],[13],[14],[15],[16],[17],[18],[19],[20],[21],[22],[23],[24]. The average age was 70.9 years, and the male-to-female ratio was 1: 2.7, which was similar to the epidemiology of RA patients. The patients who developed MTX-LPD had received MTX for 1.5 to 21 years (average 7.2 years). Regarding the site of occurrence, gingiva was the most common site in 14 patients.
Clinical findings in patients with bone exposure were similar to those of MRONJ, but at least 5 patients had not taken antiresorptive agents [4],[5],[6]. Although the mechanism of jaw osteonecrosis in MTX-LPD has not been clarified, it is considered that free radicals of MTX disrupt the mucosal barrier, and immunosuppression reduces the ability to protect against bacterial infection [25],[26]. In addition, inhibition of bone turnover by suppression of osteoclast differentiation and osteoblast proliferation, and inhibition of angiogenesis because of MTX may also be involved in osteonecrosis [27],[28],[29]. Recently, there have been several reports of ONJ caused by MTX in the absence of lymphoproliferative disorder or antiresorptive and antiangiogenic medications [30]. In our case, it could be considered that immunosuppression-induced bacterial infection after bone exposure due to soft tissue ulcer may have caused osteonecrosis, since findings of lymphoma invasion in the bone were not observed.
Immediate discontinuation of MTX and concomitant immunosuppressive drugs leads to regression in half of the patients with suspected MTX-LPD [2],[7]. Moreover, approximately 80% of MTX-LPD cases occurring in the oral region have demonstrated regression by MTX discontinuation (Table 1). Ichikawa et al. reported that Epstein–Barr virus (EBV) was detected in 60% of MTX-LPD cases, and 85% of EBV-positive cases showed spontaneous regression by withdrawing MTX [7]. Patients with spontaneous regression showed significantly higher EBV positivity compared to those without spontaneous regression. In our case, despite EBV-positivity, the disease progressed without regression following MTX withdrawal. It was considered that risk factors, including stage IV-A of Ann Arbor classification, age >62 years, LDH 431 U/L, pathologic Ki-69 expression in 80% of atypical cells, and numerous mitotic figures might be the reasons for progression of the lesion [31],[32].
Furthermore, in our case, bleeding equivalent to class III as per the ATLS classification was observed because of progression of the lesion [8]. The pterygoid venous plexus and posterior superior alveolar artery are located around the upper molars and maxillary tubercle, and these were considered to be the causative vessels for bleeding. Activation of microangiogenesis by the disease, oral administration of cilostazol for previous myocardial infarction, and the vulnerability of the oral cavity to the external environment were also considered as plausible causes of bleeding. Although hemostasis was achieved by using microfibrous collagen hemostatic material and tie-over under local anesthesia in this case, surgical ligation or catheter embolization may be considered if hemostasis is difficult. Generally, treatment of osteonecrosis is performed conservatively in all cases of MTX-LPD [6],[15],[16],[23]. In our case, conservative therapy was prioritized for continued treatment of DLBCL, and sequestrectomy was performed under local anesthesia because of separation of the sequestrum after 3 courses of R-COP. Treatment of ONJ should be considered according to the general condition of the individual patient and the status of MTX-LPD treatment.
Conclusion
Approximately 80% of MTX-LPD cases occurring in the oral region show regression after MTX discontinuation, but we experienced a case in which bleeding occurred with progression of the lesion without spontaneous regression after discontinuation. Careful follow-up is required during the MTX discontinuation period.
REFERENCES
1.
Kameda H, Fujii T, Nakajima A, et al. Japan College of Rheumatology guideline for the use of methotrexate in patients with rheumatoid arthritis. Mod Rheumatol 2019;29(1):31–40. [CrossRef]
[Pubmed]

2.
Jaffe ES, Campo E, Harris NL, Pileri SA. Stein H, Swerdlow SH. Introduction and overview of the classification of the lymphoid neoplasms. In: Swerdlow SH, Campo E, Harris NL, Jaffe ES, Pileri SA, Stein H, Thiele J, editors. World Health Organization Classification of Tumours of Haematopoietic and Lymphoid Tissues, revised 4ed. Lyon: IARC Press; 2017. p. 190–8.

3.
Ruggiero SL, Dodson TB, Fantasia J, et al. American Association of Oral and Maxillofacial Surgeons position paper on medication-related osteonecrosis of the jaw–2014 update. J Oral Maxillofac Surg 2014;72(10):1938–56. [CrossRef]
[Pubmed]

4.
Tanaka A, Shigematsu H, Kojima M, Sakashita H, Kusama K. Methotrexate-associated lymphoproliferative disorder arising in a patient with adult still's disease. J Oral Maxillofac Surg 2008;66(7):1492–5. [CrossRef]
[Pubmed]

5.
Horie N, Kawano R, Kaneko T, Shimoyama T. Methotrexate-related lymphoproliferative disorder arising in the gingiva of a patient with rheumatoid arthritis. Aust Dent J 2015;60(3):408–11. [CrossRef]
[Pubmed]

6.
Furudate K, Satake A, Narita N, Kobayashi W. Methotrexate-related lymphoproliferative disorder in patients with osteonecrosis of the jaw: A 3-case report and literature review. J Oral Maxillofac Surg 2018;76(1):97–111. [CrossRef]
[Pubmed]

7.
Ichikawa A, Arakawa F, Kiyasu J, et al. Methotrexate/iatrogenic lymphoproliferative disorders in rheumatoid arthritis: Histology, Epstein-Barr virus, and clonality are important predictors of disease progression and regression. Eur J Haematol 2013;91(1):20–8. [CrossRef]
[Pubmed]

8.
9.
Ellman MH, Hurwitz H, Thomas C, Kozloff M. Lymphoma developing in a patient with rheumatoid arthritis taking low dose weekly methotrexate. J Rheumatol 1991;18(11):1741–3.
[Pubmed]

10.
Kalantzis A, Marshman Z, Falconer DT, Morgan PR, Odell EW. Oral effects of low-dose methotrexate treatment. Oral Surg Oral Med Oral Pathol Oral Radiol Endod 2005;100(1):52–62. [CrossRef]
[Pubmed]

11.
Kojima M, Itoh H, Hirabayashi K, et al. Methotrexate-associated lymphoproliferative disorders. A clinicopathological study of 13 Japanese cases. Pathol Res Pract 2006;202(9):679–85. [CrossRef]
[Pubmed]

12.
Uneda S, Sonoki T, Nakamura Y, Matsuoka H, Nakakuma H. Rapid vanishing of tumors by withdrawal of methotrexate in Epstein-Barr virus-related B cell lymphoproliferative disorder. Intern Med 2008;47(15):1445–6. [CrossRef]
[Pubmed]

13.
Kikuchi K, Miyazaki Y, Tanaka A, et al. Methotrexate-related Epstein-Barr virus (EBV)-associated lymphoproliferative disorder--so-called [CrossRef]
[Pubmed]

14.
Ishida M, Hodohara K, Yoshii M, et al. Methotrexate-related Epstein-Barr virus-associated lymphoproliferative disorder occurring in the gingiva of a patient with rheumatoid arthritis. Int J Clin Exp Pathol 2013;6(10):2237–41.
[Pubmed]

15.
16.
Kudoh M, Harada H, Matsumoto K, Sato Y, Omura K, Ishii Y. Methotrexate-associated lymphoproliferative disorder arising in the retromolar triangle and lung of a patient with rheumatoid arthritis. Oral Surg Oral Med Oral Pathol Oral Radiol 2014;118(4):e105–10. [CrossRef]
[Pubmed]

17.
Kawano N, Ono N, Kawano S, et al. Clinical features and outcomes of 9 patients with immunodeficiency-associated lymphoproliferative disorders treated at a single institution. J Clin Exp Hematop 2014;54(3):187–96. [CrossRef]
[Pubmed]

18.
19.
Kikuchi K, Ishige T, Ide F, et al. Overexpression of activation-induced cytidine deaminase in MTX- and age-related Epstein-Barr virus-associated B-cell lymphoproliferative disorders of the head and neck. J Oncol 2015;2015:605750. [CrossRef]
[Pubmed]

20.
Hashimoto K, Nagao T, Saito T, Kinoshita H. Methotrexate-associated lymphoproliferative disorders of the tongue developing in patients with rheumatoid arthritis: A report of 2 cases and a review. Oral Surg Oral Med Oral Pathol Oral Radiol 2015;119(1):e1–5. [CrossRef]
[Pubmed]

21.
22.
Mishima S, Takahashi K, Tomioka T, Bessho K. Numb chin syndrome as initial manifestation of bisphosphonate-related osteomyelitis of the jaw and methotrexate-associated lymphoproliferative disorders: A rare case. Br J Oral Maxillofac Surg 2016;54(1):114–5. [CrossRef]
[Pubmed]

23.
Furukawa S, Oobu K, Moriyama M, et al. Oral methotrexate-related lymphoproliferative disease presenting with severe osteonecrosis of the jaw: A case report and literature review. Intern Med 2018;57(4):575–81. [CrossRef]
[Pubmed]

24.
25.
Peterson DE. Oral toxicity of chemotherapeutic agents. Semin Oncol 1992;19(5):478–91.
[Pubmed]

26.
Gressier B, Lebegue S, Brunet C, et al. Pro-oxidant properties of methotrexate: Evaluation and prevention by an anti-oxidant drug. Pharmazie 1994;49(9):679–81.
[Pubmed]

27.
Annussek T, Kleinheinz J, Thomas S, Joos U, Wermker K. Short time administration of antirheumatic drugs – methotrexate as a strong inhibitor of osteoblast's proliferation in vitro. Head Face Med 2012;8:26. [CrossRef]
[Pubmed]

28.
Kanagawa H, Masuyama R, Morita M, et al. Methotrexate inhibits osteoclastogenesis by decreasing RANKL-induced calcium influx into osteoclast progenitors. J Bone Miner Metab 2016;34(5):526–31. [CrossRef]
[Pubmed]

29.
Hirata S, Matsubara T, Saura R, Tateishi H, Hirohata K. Inhibition of in vitro vascular endothelial cell proliferation and in vivo neovascularization by low-dose methotrexate. Arthritis Rheum 1989;32(9):1065–73. [CrossRef]
[Pubmed]

30.
Henien M, Carey B, Hullah E, Sproat C, Patel V. Methotrexate-associated osteonecrosis of the jaw: A report of two cases. Oral Surg Oral Med Oral Pathol Oral Radiol 2017;124(6):e283–7. [CrossRef]
[Pubmed]

31.
Song MK, Chung JS, Lee JJ, et al. High Ki-67 expression in involved bone marrow predicts worse clinical outcome in diffuse large B cell lymphoma patients treated with R-CHOP therapy. Int J Hematol 2015;101(2):140–7. [CrossRef]
[Pubmed]

32.
Zhou Z, Sehn LH, Rademaker AW, et al. An enhanced International Prognostic Index (NCCN-IPI) for patients with diffuse large B-cell lymphoma treated in the rituximab era. Blood 2014;123(6):837–42. [CrossRef]
[Pubmed]

SUPPORTING INFORMATION
Author Contributions
Moemi Kimura - Conception of the work, Design of the work, Acquisition of data, Analysis of data, Drafting the work, Revising the work critically for important intellectual content, Final approval of the version to be published, Agree to be accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved.
Takazumi Yasui - Conception of the work, Design of the work, Acquisition of data, Analysis of data, Drafting the work, Revising the work critically for important intellectual content, Final approval of the version to be published, Agree to be accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved.
Hiroki Nagamine - Conception of the work, Design of the work, Acquisition of data, Analysis of data, Drafting the work, Revising the work critically for important intellectual content, Final approval of the version to be published, Agree to be accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved.
Shosuke Yajima - Conception of the work, Design of the work, Acquisition of data, Analysis of data, Drafting the work, Revising the work critically for important intellectual content, Final approval of the version to be published, Agree to be accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved.
Rie Kodaka - Conception of the work, Design of the work, Acquisition of data, Analysis of data, Drafting the work, Revising the work critically for important intellectual content, Final approval of the version to be published, Agree to be accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved.
Katsuhiro Onizawa - Conception of the work, Design of the work, Acquisition of data, Analysis of data, Drafting the work, Revising the work critically for important intellectual content, Final approval of the version to be published, Agree to be accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved.
Guarantor of SubmissionThe corresponding author is the guarantor of submission.
Source of SupportNone
Consent StatementWritten informed consent was obtained from the patient for publication of this article.
Data AvailabilityAll relevant data are within the paper and its Supporting Information files.
Conflict of InterestAuthors declare no conflict of interest.
Copyright© 2021 Moemi Kimura et al. This article is distributed under the terms of Creative Commons Attribution License which permits unrestricted use, distribution and reproduction in any medium provided the original author(s) and original publisher are properly credited. Please see the copyright policy on the journal website for more information.